Radiogenomics: using genetics to identify cancer patients at risk for development of adverse effects following radiotherapy
- PMID: 24441285
- PMCID: PMC3946319
- DOI: 10.1158/2159-8290.CD-13-0197
Radiogenomics: using genetics to identify cancer patients at risk for development of adverse effects following radiotherapy
Abstract
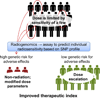
Normal-tissue adverse effects following radiotherapy are common and significantly affect quality of life. These effects cannot be accounted for by dosimetric, treatment, or demographic factors alone, and evidence suggests that common genetic variants are associated with radiotherapy adverse effects. The field of radiogenomics has evolved to identify such genetic risk factors. Radiogenomics has two goals: (i) to develop an assay to predict which patients with cancer are most likely to develop radiation injuries resulting from radiotherapy, and (ii) to obtain information about the molecular pathways responsible for radiation-induced normal-tissue toxicities. This review summarizes the history of the field and current research.
Significance: A single-nucleotide polymorphism–based predictive assay could be used, along with clinical and treatment factors, to estimate the risk that a patient with cancer will develop adverse effects from radiotherapy. Such an assay could be used to personalize therapy and improve quality of life for patients with cancer.
2014 AACR
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures


References
-
- Boyle P, Levin B. World Cancer Report 2008: World Health Organization. 2008
-
- Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–1145. - PubMed
-
- Aziz NM. Cancer survivorship research: state of knowledge, challenges and opportunities. Acta Oncol. 2007;46:417–432. - PubMed
-
- Vale C, Nightingale A, Spera N, Whelan A, Hanley B, Tierney JF. Late complications from chemoradiotherapy for cervical cancer: reflections from cervical cancer survivors 10 years after the national cancer institute alert. Clin Oncol (R Coll Radiol) 2010;22:588–589. - PubMed
-
- Miller RC, Schwartz DJ, Sloan JA, Griffin PC, Deming RL, Anders JC, et al. Mometasone furoate effect on acute skin toxicity in breast cancer patients receiving radiotherapy: a phase III double-blind, randomized trial from the North Central Cancer Treatment Group N06C4. Int J Radiat Oncol Biol Phys. 2011;79:1460–1466. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

