In vitro and in vivo bioactivity assessment of a polylactic acid/hydroxyapatite composite for bone regeneration
- PMID: 24441389
- PMCID: PMC3961484
- DOI: 10.4161/biom.27664
In vitro and in vivo bioactivity assessment of a polylactic acid/hydroxyapatite composite for bone regeneration
Abstract
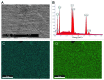
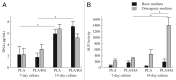
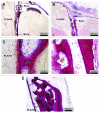
Synthetic bone graft substitutes based on composites consisting of a polymer and a calcium-phosphate (CaP) ceramic are developed with the aim to satisfy both mechanical and bioactivity requirements for successful bone regeneration. In the present study, we have employed extrusion to produce a composite consisting of 50 wt.% poly(D,L-lactic acid) (PLA) and 50 wt.% nano-sized hydroxyapatite (HA) powder, achieving homogeneous distribution of the ceramic within the polymeric phase. In vitro, in both a simulated physiological saline (SPS) and a simulated body fluid (SBF), a greater weight loss was observed for PLA/HA than for PLA particles upon 12-week immersion. Furthermore, in SPS, a continuous release of calcium and phosphate from the composite was measured, whereas in SBF, decrease of the amount of the two ions in the solution was observed both for PLA and PLA/HA accompanied with the formation of a CaP layer on the surface. In vitro characterization of the composite bioactivity was performed by culturing human mesenchymal stromal cells (hMSCs) and assessing proliferation and osteogenic differentiation, with PLA as a control. Both PLA/HA composite and PLA control were shown to support hMSCs proliferation over a period of two weeks. In addition, the composite significantly enhanced alkaline phosphatase (ALP) activity of hMSCs in osteogenic medium as compared with the polymer control. A novel implant design was employed to develop implants from dense, extruded materials, suitable for testing osteoinductivity in vivo. In a preliminary study in dogs, PLA/HA composite implants induced heterotopic bone formation upon 12-week intramuscular implantation in all animals, in contrast to PLA control, which was not osteoinductive. Unlike in vitro, a more pronounced degradation of PLA was observed in vivo as compared with PLA/HA composite.
Keywords: bone regeneration; composite; hydroxyapatite; osteoinduction; polylactic acid.
Figures




Similar articles
-
Independent effects of the chemical and microstructural surface properties of polymer/ceramic composites on proliferation and osteogenic differentiation of human MSCs.Acta Biomater. 2016 Sep 15;42:364-377. doi: 10.1016/j.actbio.2016.06.018. Epub 2016 Jun 16. Acta Biomater. 2016. PMID: 27318269
-
Monolithic calcium phosphate/poly(lactic acid) composite versus calcium phosphate-coated poly(lactic acid) for support of osteogenic differentiation of human mesenchymal stromal cells.J Mater Sci Mater Med. 2016 Mar;27(3):54. doi: 10.1007/s10856-016-5666-9. Epub 2016 Jan 19. J Mater Sci Mater Med. 2016. PMID: 26787486 Free PMC article.
-
Biocompatibility and bone-repairing effects: comparison between porous poly-lactic-co-glycolic acid and nano-hydroxyapatite/poly(lactic acid) scaffolds.J Biomed Nanotechnol. 2014 Jun;10(6):1091-104. doi: 10.1166/jbn.2014.1696. J Biomed Nanotechnol. 2014. PMID: 24749403
-
Biomaterials for periodontal regeneration: a review of ceramics and polymers.Biomatter. 2012 Oct-Dec;2(4):271-7. doi: 10.4161/biom.22948. Biomatter. 2012. PMID: 23507891 Free PMC article. Review.
-
Review paper: behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition.J Biomater Appl. 2008 Nov;23(3):197-212. doi: 10.1177/0885328208096798. J Biomater Appl. 2008. PMID: 18996965 Review.
Cited by
-
Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering.Adv Drug Deliv Rev. 2016 Dec 15;107:247-276. doi: 10.1016/j.addr.2016.04.015. Epub 2016 Apr 25. Adv Drug Deliv Rev. 2016. PMID: 27125191 Free PMC article. Review.
-
Mechanical Properties and Bioactivity of Poly(Lactic Acid) Composites Containing Poly(Glycolic Acid) Fiber and Hydroxyapatite Particles.Nanomaterials (Basel). 2021 Jan 18;11(1):249. doi: 10.3390/nano11010249. Nanomaterials (Basel). 2021. PMID: 33477735 Free PMC article.
-
Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments.Molecules. 2021 May 18;26(10):3007. doi: 10.3390/molecules26103007. Molecules. 2021. PMID: 34070157 Free PMC article. Review.
-
Comparison of the Efficacy of Three Different Bone Regeneration Materials: An Animal Study.Eur J Dent. 2019 Feb;13(1):22-28. doi: 10.1055/s-0039-1688735. Epub 2019 Jun 6. Eur J Dent. 2019. PMID: 31170752 Free PMC article.
-
Bioglasses Versus Bioactive Calcium Phosphate Derivatives as Advanced Ceramics in Tissue Engineering: Comparative and Comprehensive Study, Current Trends, and Innovative Solutions.J Funct Biomater. 2025 May 3;16(5):161. doi: 10.3390/jfb16050161. J Funct Biomater. 2025. PMID: 40422826 Free PMC article. Review.
References
-
- Davies JE, Hosseini MM. Histodynamic of endosseous wound healing. In: Davies JE, editor. Toronto, Canada: Em squared Inc.; 2000. p. 1-14.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
