G protein-coupled receptors: what a difference a 'partner' makes
- PMID: 24441568
- PMCID: PMC3907859
- DOI: 10.3390/ijms15011112
G protein-coupled receptors: what a difference a 'partner' makes
Abstract
G protein-coupled receptors (GPCRs) are important cell signaling mediators, involved in essential physiological processes. GPCRs respond to a wide variety of ligands from light to large macromolecules, including hormones and small peptides. Unfortunately, mutations and dysregulation of GPCRs that induce a loss of function or alter expression can lead to disorders that are sometimes lethal. Therefore, the expression, trafficking, signaling and desensitization of GPCRs must be tightly regulated by different cellular systems to prevent disease. Although there is substantial knowledge regarding the mechanisms that regulate the desensitization and down-regulation of GPCRs, less is known about the mechanisms that regulate the trafficking and cell-surface expression of newly synthesized GPCRs. More recently, there is accumulating evidence that suggests certain GPCRs are able to interact with specific proteins that can completely change their fate and function. These interactions add on another level of regulation and flexibility between different tissue/cell-types. Here, we review some of the main interacting proteins of GPCRs. A greater understanding of the mechanisms regulating their interactions may lead to the discovery of new drug targets for therapy.
Figures
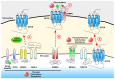
 represents glycosylation.
represents glycosylation.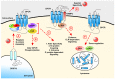
References
-
- Fredriksson R., Lagerstrom M.C., Lundin L.G., Schioth H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256–1272. - PubMed
-
- Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: Molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol. Ther. 2004;103:21–80. - PubMed
-
- Ellgaard L., Molinari M., Helenius A. Setting the standards: Quality control in the secretory pathway. Science. 1999;286:1882–1888. - PubMed
-
- Petaja-Repo U.E., Hogue M., Laperriere A., Bhalla S., Walker P., Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J. Biol. Chem. 2001;276:4416–4423. - PubMed
-
- Cook L.B., Zhu C.C., Hinkle P.M. Thyrotropin-releasing hormone receptor processing: Role of ubiquitination and proteasomal degradation. Mol. Endocrinol. 2003;17:1777–1791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

