Medial prefrontal D1 dopamine neurons control food intake
- PMID: 24441680
- PMCID: PMC3968853
- DOI: 10.1038/nn.3625
Medial prefrontal D1 dopamine neurons control food intake
Abstract
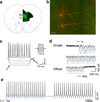
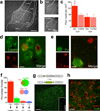
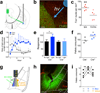
Although the prefrontal cortex influences motivated behavior, its role in food intake remains unclear. Here, we demonstrate a role for D1-type dopamine receptor-expressing neurons in the medial prefrontal cortex (mPFC) in the regulation of feeding. Food intake increases activity in D1 neurons of the mPFC in mice, and optogenetic photostimulation of D1 neurons increases feeding. Conversely, inhibition of D1 neurons decreases intake. Stimulation-based mapping of prefrontal D1 neuron projections implicates the medial basolateral amygdala (mBLA) as a downstream target of these afferents. mBLA neurons activated by prefrontal D1 stimulation are CaMKII positive and closely juxtaposed to prefrontal D1 axon terminals. Finally, photostimulating these axons in the mBLA is sufficient to increase feeding, recapitulating the effects of mPFC D1 stimulation. These data describe a new circuit for top-down control of food intake.
Figures


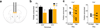
References
-
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:176–202. - PubMed
-
- de Araujo IE, Rolls E, Kringelbach M, Mcglone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur. J. Neurosci. 2003;18:2059–2068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

