Transformation of taxol-stabilized microtubules into inverted tubulin tubules triggered by a tubulin conformation switch
- PMID: 24441880
- PMCID: PMC3946914
- DOI: 10.1038/nmat3858
Transformation of taxol-stabilized microtubules into inverted tubulin tubules triggered by a tubulin conformation switch
Abstract
Bundles of taxol-stabilized microtubules (MTs)--hollow tubules comprised of assembled αβ-tubulin heterodimers--spontaneously assemble above a critical concentration of tetravalent spermine and are stable over long times at room temperature. Here we report that at concentrations of spermine several-fold higher the MT bundles (B(MT)) quickly become unstable and undergo a shape transformation to bundles of inverted tubulin tubules (B(ITT)), the outside surface of which corresponds to the inner surface of the B(MT) tubules. Using transmission electron microscopy and synchrotron small-angle X-ray scattering, we quantitatively determined both the nature of the B(MT)-to-B(ITT) transformation pathway, which results from a spermine-triggered conformation switch from straight to curved in the constituent taxol-stabilized tubulin oligomers, and the structure of the B(ITT) phase, which is formed of tubules of helical tubulin oligomers. Inverted tubulin tubules provide a platform for studies requiring exposure and availability of the inside, luminal surface of MTs to MT-targeted drugs and MT-associated proteins.
Figures



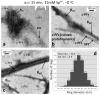
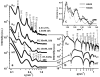
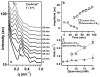
Comment in
-
Materials science. How shape affects microtubule and nanoparticle assembly.Science. 2014 Feb 28;343(6174):981-2. doi: 10.1126/science.1250827. Science. 2014. PMID: 24578572 No abstract available.
References
-
- Bray D. Cell Movements: From Molecules to Motility. 2 Garland; New York: 2001.
-
- Thomas TD, Earnshaw WC, Lippincott-Schwartz J. Cell Biology. 2 Saunders, Elsevier; Philadelphia: 2008.
-
- Israelachvili JN. Intermolecular & Surface Forces. Academic Press; Waltham: 1992.
-
- Sackmann E. Membrane Bending Energy Concept of Vesicle-Shape and Cell-Shape and Shape-Transitions. Febs. Letters. 1994;346:3–16. - PubMed
-
- Chiruvolu S, et al. A Phase of Liposomes with Entagled Tubular Vesicles. Science. 1994;266:1222–1225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

