Dopamine in the dorsal hippocampus impairs the late consolidation of cocaine-associated memory
- PMID: 24442095
- PMCID: PMC4023137
- DOI: 10.1038/npp.2014.11
Dopamine in the dorsal hippocampus impairs the late consolidation of cocaine-associated memory
Abstract
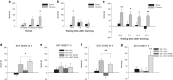
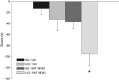
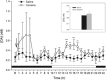
Cocaine is thought to be addictive because it elevates dopamine levels in the striatum, reinforcing drug-seeking habits. Cocaine also elevates dopamine levels in the hippocampus, a structure involved in contextual conditioning as well as in reward function. Hippocampal dopamine promotes the late phase of consolidation of an aversive step-down avoidance memory. Here, we examined the role of hippocampal dopamine function in the persistence of the conditioned increase in preference for a cocaine-associated compartment. Blocking dorsal hippocampal D1-type receptors (D1Rs) but not D2-type receptors (D2Rs) 12 h after a single training trial extended persistence of the normally short-lived memory; conversely, a general and a specific phospholipase C-coupled D1R agonist (but not a D2R or adenylyl cyclase-coupled D1R agonist) decreased the persistence of the normally long-lived memory established by three-trial training. These effects of D1 agents were opposite to those previously established in a step-down avoidance task, and were here also found to be opposite to those in a lithium chloride-conditioned avoidance task. After returning to normal following cocaine injection, dopamine levels in the dorsal hippocampus were found elevated again at the time when dopamine antagonists and agonists were effective: between 13 and 17 h after cocaine injection. These findings confirm that, long after the making of a cocaine-place association, hippocampal activity modulates memory consolidation for that association via a dopamine-dependent mechanism. They suggest a dynamic role for dorsal hippocampal dopamine in this late-phase memory consolidation and, unexpectedly, differential roles for late consolidation of memories for places that induce approach or withdrawal because of a drug association.
Figures
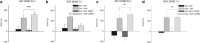

References
-
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua RL, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. - PubMed
-
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. - PubMed
-
- Busse GD, Riley AL. Cocaine, but not alcohol, reinstates cocaine-induced place preferences. Pharmacol Biochem Behav. 2004;78:827–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

