Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100
- PMID: 24442096
- PMCID: PMC4023154
- DOI: 10.1038/npp.2014.2
Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100
Abstract
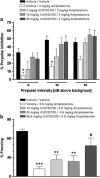
Accumulating evidence suggests that selective M4 muscarinic acetylcholine receptor (mAChR) activators may offer a novel strategy for the treatment of psychosis. However, previous efforts to develop selective M4 activators were unsuccessful because of the lack of M4 mAChR subtype specificity and off-target muscarinic adverse effects. We recently developed VU0152100, a highly selective M4 positive allosteric modulator (PAM) that exerts central effects after systemic administration. We now report that VU0152100 dose-dependently reverses amphetamine-induced hyperlocomotion in rats and wild-type mice, but not in M4 KO mice. VU0152100 also blocks amphetamine-induced disruption of the acquisition of contextual fear conditioning and prepulse inhibition of the acoustic startle reflex. These effects were observed at doses that do not produce catalepsy or peripheral adverse effects associated with non-selective mAChR agonists. To further understand the effects of selective potentiation of M4 on region-specific brain activation, VU0152100 alone and in combination with amphetamine were evaluated using pharmacologic magnetic resonance imaging (phMRI). Key neural substrates of M4-mediated modulation of the amphetamine response included the nucleus accumbens (NAS), caudate-putamen (CP), hippocampus, and medial thalamus. Functional connectivity analysis of phMRI data, specifically assessing correlations in activation between regions, revealed several brain networks involved in the M4 modulation of amphetamine-induced brain activation, including the NAS and retrosplenial cortex with motor cortex, hippocampus, and medial thalamus. Using in vivo microdialysis, we found that VU0152100 reversed amphetamine-induced increases in extracellular dopamine levels in NAS and CP. The present data are consistent with an antipsychotic drug-like profile of activity for VU0152100. Taken together, these data support the development of selective M4 PAMs as a new approach to the treatment of psychosis and cognitive impairments associated with psychiatric disorders such as schizophrenia.
Figures




References
-
- American Psychiatric Association 2000Diagnostic and Statistical Manual of Mental Disorders4th edn.American Psychiatric Publishing: Washington, DC
-
- Andersen MB, Fink-Jensen A, Peacock L, Gerlach J, Bymaster F, Lundbaek JA, et al. The muscarinic M1/M4 receptor agonist xanomeline exhibits antipsychotic-like activity in the Cebus apella monkey. Neuropsychopharmacology. 2003;28:1168–1175. - PubMed
-
- Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. - PubMed
-
- Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. - PubMed
-
- Bonner TI, Young AC, Brann MR, Buckley NJ. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988;1:403–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

