Characterization of T-cell responses to cryptic epitopes in recipients of a noncodon-optimized HIV-1 vaccine
- PMID: 24442221
- PMCID: PMC3896890
- DOI: 10.1097/QAI.0b013e3182a9917e
Characterization of T-cell responses to cryptic epitopes in recipients of a noncodon-optimized HIV-1 vaccine
Abstract
Introduction: Cryptic epitopes (CEs) can be encoded by any of the 5 alternative reading frames (ARFs, 2 sense and 3 antisense) of a known gene. Although CE responses are commonly detected during HIV-1 infection, it is not known whether these responses are induced after vaccination.
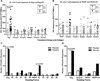
Methods: Using a bioinformatic approach, we determined that vaccines with codon-optimized HIV inserts significantly skewed CE sequences and are not likely to induce crossreactive responses to natural HIV CE. We then evaluated the CE- and protein-specific T-cell responses using Gag, Pol, and ARF peptide pools among participants immunized with a non-codon optimized vaccine regimen of 2 pGA2/JS7 DNA primes followed by 2 MVA/HIV62 Gag-Pol-Env vector boosts or 4 saline injections.
Results: Vaccinees had significantly more interferon gamma enzyme-linked immunosorbent spot (IFNγ ELISpot) responses toward Gag (P = 0.003) but not toward Pol protein than did placebo recipients. However, CE-specific T-cell responses were low in magnitude, and their frequencies did not differ significantly between vaccine and placebo recipients. Additionally, most positive CE responses could not be mapped to individual peptides. After expanding responses in a cultured assay, however, the frequency and the median magnitude of responses to ARF peptides were significantly greater in vaccinees (P < 0.0001), indicating that CE-specific T-cell responses are present but below an ex vivo assay's limit of detection.
Conclusions: Our data demonstrate that HIV-1 vaccines currently in clinical trials are poorly immunogenic with regard to CE-specific T-cell responses. Therefore, the context of HIV-1 immunogens may need to be modified as a comprehensive strategy to broaden vaccine-induced T-cell responses.
Conflict of interest statement
Figures



References
-
- Taylor BS, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008;359:1965–1966. - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

