Neuronal calcium signaling: function and dysfunction
- PMID: 24442513
- PMCID: PMC11113927
- DOI: 10.1007/s00018-013-1550-7
Neuronal calcium signaling: function and dysfunction
Abstract
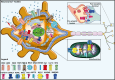
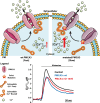
Calcium (Ca(2+)) is an universal second messenger that regulates the most important activities of all eukaryotic cells. It is of critical importance to neurons as it participates in the transmission of the depolarizing signal and contributes to synaptic activity. Neurons have thus developed extensive and intricate Ca(2+) signaling pathways to couple the Ca(2+) signal to their biochemical machinery. Ca(2+) influx into neurons occurs through plasma membrane receptors and voltage-dependent ion channels. The release of Ca(2+) from the intracellular stores, such as the endoplasmic reticulum, by intracellular channels also contributes to the elevation of cytosolic Ca(2+). Inside the cell, Ca(2+) is controlled by the buffering action of cytosolic Ca(2+)-binding proteins and by its uptake and release by mitochondria. The uptake of Ca(2+) in the mitochondrial matrix stimulates the citric acid cycle, thus enhancing ATP production and the removal of Ca(2+) from the cytosol by the ATP-driven pumps in the endoplasmic reticulum and the plasma membrane. A Na(+)/Ca(2+) exchanger in the plasma membrane also participates in the control of neuronal Ca(2+). The impaired ability of neurons to maintain an adequate energy level may impact Ca(2+) signaling: this occurs during aging and in neurodegenerative disease processes. The focus of this review is on neuronal Ca(2+) signaling and its involvement in synaptic signaling processes, neuronal energy metabolism, and neurotransmission. The contribution of altered Ca(2+) signaling in the most important neurological disorders will then be considered.
Figures



References
-
- Carafoli E, Malmstrom K, Sigel E, Crompton M. The regulation of intracellular calcium. Clin Endocrinol (Oxf) 1976;5(Suppl):49S–59S. - PubMed
-
- Brini M, Cali T, Ottolini D, Carafoli E. Intracellular calcium homeostasis and signaling. Met Ions Life Sci. 2013;12:119–168. - PubMed
-
- Mellstrom B, Savignac M, Gomez-Villafuertes R, Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev. 2008;88(2):421–449. - PubMed
-
- Carafoli E. The unusual history and unique properties of the calcium signal. In: Krebs J, Michalak M, editors. Calcium: a matter of life and death. Amsterdam: Elsevier; 2007. pp. 3–22.
-
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

