Molecular crowders and cosolutes promote folding cooperativity of RNA under physiological ionic conditions
- PMID: 24442612
- PMCID: PMC3923128
- DOI: 10.1261/rna.042747.113
Molecular crowders and cosolutes promote folding cooperativity of RNA under physiological ionic conditions
Abstract
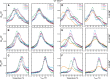
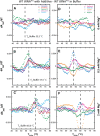
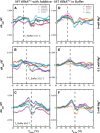
Folding mechanisms of functional RNAs under idealized in vitro conditions of dilute solution and high ionic strength have been well studied. Comparatively little is known, however, about mechanisms for folding of RNA in vivo where Mg(2+) ion concentrations are low, K(+) concentrations are modest, and concentrations of macromolecular crowders and low-molecular-weight cosolutes are high. Herein, we apply a combination of biophysical and structure mapping techniques to tRNA to elucidate thermodynamic and functional principles that govern RNA folding under in vivo-like conditions. We show by thermal denaturation and SHAPE studies that tRNA folding cooperativity increases in physiologically low concentrations of Mg(2+) (0.5-2 mM) and K(+) (140 mM) if the solution is supplemented with physiological amounts (∼ 20%) of a water-soluble neutral macromolecular crowding agent such as PEG or dextran. Low-molecular-weight cosolutes show varying effects on tRNA folding cooperativity, increasing or decreasing it based on the identity of the cosolute. For those additives that increase folding cooperativity, the gain is manifested in sharpened two-state-like folding transitions for full-length tRNA over its secondary structural elements. Temperature-dependent SHAPE experiments in the absence and presence of crowders and cosolutes reveal extent of cooperative folding of tRNA on a nucleotide basis and are consistent with the melting studies. Mechanistically, crowding agents appear to promote cooperativity by stabilizing tertiary structure, while those low molecular cosolutes that promote cooperativity stabilize tertiary structure and/or destabilize secondary structure. Cooperative folding of functional RNA under physiological-like conditions parallels the behavior of many proteins and has implications for cellular RNA folding kinetics and evolution.
Keywords: biological cosolutes; folding cooperativity; macromolecular crowding; tRNA; temperature-dependent structure mapping.
Figures

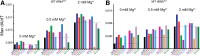




Similar articles
-
Cooperative RNA Folding under Cellular Conditions Arises From Both Tertiary Structure Stabilization and Secondary Structure Destabilization.Biochemistry. 2017 Jul 11;56(27):3422-3433. doi: 10.1021/acs.biochem.7b00325. Epub 2017 Jun 28. Biochemistry. 2017. PMID: 28657303 Free PMC article.
-
Facilitation of RNA enzyme activity in the molecular crowding media of cosolutes.J Am Chem Soc. 2009 Nov 25;131(46):16881-8. doi: 10.1021/ja9066628. J Am Chem Soc. 2009. PMID: 19874030
-
Effects of Molecular Crowders on Single-Molecule Nucleic Acid Folding: Temperature-Dependent Studies Reveal True Crowding vs Enthalpic Interactions.J Phys Chem B. 2021 Dec 9;125(48):13147-13157. doi: 10.1021/acs.jpcb.1c07852. Epub 2021 Nov 23. J Phys Chem B. 2021. PMID: 34813337
-
Probing the kinetic and thermodynamic consequences of the tetraloop/tetraloop receptor monovalent ion-binding site in P4-P6 RNA by smFRET.Biochem Soc Trans. 2015 Apr;43(2):172-8. doi: 10.1042/BST20140268. Biochem Soc Trans. 2015. PMID: 25849913 Free PMC article. Review.
-
Noncanonical structures and their thermodynamics of DNA and RNA under molecular crowding: beyond the Watson-Crick double helix.Int Rev Cell Mol Biol. 2014;307:205-73. doi: 10.1016/B978-0-12-800046-5.00008-4. Int Rev Cell Mol Biol. 2014. PMID: 24380597 Review.
Cited by
-
Salt-Dependent RNA Pseudoknot Stability: Effect of Spatial Confinement.Front Mol Biosci. 2021 Apr 13;8:666369. doi: 10.3389/fmolb.2021.666369. eCollection 2021. Front Mol Biosci. 2021. PMID: 33928126 Free PMC article.
-
Cell-Free Systems Based on CHO Cell Lysates: Optimization Strategies, Synthesis of "Difficult-to-Express" Proteins and Future Perspectives.PLoS One. 2016 Sep 29;11(9):e0163670. doi: 10.1371/journal.pone.0163670. eCollection 2016. PLoS One. 2016. PMID: 27684475 Free PMC article.
-
Direct observation of tRNA-chaperoned folding of a dynamic mRNA ensemble.Nat Commun. 2023 Sep 6;14(1):5438. doi: 10.1038/s41467-023-41155-3. Nat Commun. 2023. PMID: 37673863 Free PMC article.
-
When Phased without Water: Biophysics of Cellular Desiccation, from Biomolecules to Condensates.Chem Rev. 2023 Jul 26;123(14):9010-9035. doi: 10.1021/acs.chemrev.2c00659. Epub 2023 May 3. Chem Rev. 2023. PMID: 37132487 Free PMC article. Review.
-
Molecular Crowding Facilitates Ribozyme-Catalyzed RNA Assembly.ACS Cent Sci. 2023 Aug 3;9(8):1670-1678. doi: 10.1021/acscentsci.3c00547. eCollection 2023 Aug 23. ACS Cent Sci. 2023. PMID: 37637737 Free PMC article.
References
-
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD 1994. Molecular biology of the cell, 3rd ed Garland Science, New York
-
- Bevilacqua JM, Bevilacqua PC 1998. Thermodynamic analysis of an RNA combinatorial library contained in a short hairpin. Biochemistry 37: 15877–15884 - PubMed
-
- Blose JM, Silverman SK, Bevilacqua PC 2007. A simple molecular model for thermophilic adaptation of functional nucleic acids. Biochemistry 46: 4232–4240 - PubMed
-
- Brion P, Westhof E 1997. Hierarchy and dynamics of RNA folding. Annu Rev Biophys Biomol Struct 26: 113–137 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
