Synthesis, spectral characterization, and in vitro cellular activities of metapristone, a potential cancer metastatic chemopreventive agent derived from mifepristone (RU486)
- PMID: 24442753
- PMCID: PMC3933578
- DOI: 10.1208/s12248-013-9559-2
Synthesis, spectral characterization, and in vitro cellular activities of metapristone, a potential cancer metastatic chemopreventive agent derived from mifepristone (RU486)
Abstract
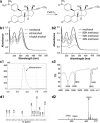
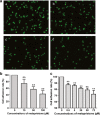
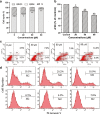
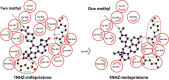
Mifepristone (RU486) is marketed and used widely by women as an abortifacient, and experimentally for psychotic depression and anticancer treatments. After administration, metapristone is found to be the most predominant metabolite of mifepristone. We hypothesized that adhesion of circulating tumor cells (CTCs) to vascular endothelial bed is a crucial starting point in metastatic cascade, and that metapristone can serve as a cancer metastatic chemopreventive agent that can interrupt adhesion and invasion of CTCs to the intima of microvasculature. In the present study, we modified the synthesis procedure to produce grams of metapristone, fully characterized its spectral properties and in vitro cellular activities, including its cytostatic effects, cell cycle arrest, mitochondrial membrane potential, and apoptosis on human colorectal cancer HT-29 cells. Metapristone concentration dependently interrupted adhesion of HT-29 cells to endothelial cells. Metapristone may potentially be a useful agent to interrupt metastatic initiation.
Figures

Similar articles
-
In vitro and in vivo efficacy and safety evaluation of metapristone and mifepristone as cancer metastatic chemopreventive agents.Biomed Pharmacother. 2016 Mar;78:291-300. doi: 10.1016/j.biopha.2016.01.017. Epub 2016 Feb 4. Biomed Pharmacother. 2016. PMID: 26898454
-
Metapristone (RU486 derivative) inhibits cell proliferation and migration as melanoma metastatic chemopreventive agent.Biomed Pharmacother. 2017 Jun;90:339-349. doi: 10.1016/j.biopha.2017.03.076. Epub 2017 Apr 2. Biomed Pharmacother. 2017. PMID: 28376402
-
Abortifacient metapristone (RU486 derivative) interrupts CXCL12/CXCR4 axis for ovarian metastatic chemoprevention.Mol Carcinog. 2017 Aug;56(8):1896-1908. doi: 10.1002/mc.22645. Epub 2017 Mar 30. Mol Carcinog. 2017. PMID: 28277622
-
The unique pharmacological characteristics of mifepristone (RU486): from terminating pregnancy to preventing cancer metastasis.Med Res Rev. 2014 Sep;34(5):979-1000. doi: 10.1002/med.21311. Epub 2014 Mar 1. Med Res Rev. 2014. PMID: 24585714 Review.
-
Similarities and differences between embryonic implantation and CTC invasion: Exploring the roles of abortifacients in cancer metastasis chemoprevention.Eur J Med Chem. 2022 Jul 5;237:114416. doi: 10.1016/j.ejmech.2022.114416. Epub 2022 Apr 26. Eur J Med Chem. 2022. PMID: 35500473 Review.
Cited by
-
Mifepristone Treatment Promotes Testicular Leydig Cell Tumor Progression in Transgenic Mice.Cancers (Basel). 2020 Nov 4;12(11):3263. doi: 10.3390/cancers12113263. Cancers (Basel). 2020. PMID: 33158280 Free PMC article.
-
Synergism of ursolic acid derivative US597 with 2-deoxy-D-glucose to preferentially induce tumor cell death by dual-targeting of apoptosis and glycolysis.Sci Rep. 2014 May 21;4:5006. doi: 10.1038/srep05006. Sci Rep. 2014. PMID: 25833312 Free PMC article.
-
A novel co-drug of aspirin and ursolic acid interrupts adhesion, invasion and migration of cancer cells to vascular endothelium via regulating EMT and EGFR-mediated signaling pathways: multiple targets for cancer metastasis prevention and treatment.Oncotarget. 2016 Nov 8;7(45):73114-73129. doi: 10.18632/oncotarget.12232. Oncotarget. 2016. PMID: 27683033 Free PMC article.
-
Systems pharmacology of mifepristone (RU486) reveals its 47 hub targets and network: comprehensive analysis and pharmacological focus on FAK-Src-Paxillin complex.Sci Rep. 2015 Jan 19;5:7830. doi: 10.1038/srep07830. Sci Rep. 2015. PMID: 25597938 Free PMC article.
-
Nitric oxide inhibits hetero-adhesion of cancer cells to endothelial cells: restraining circulating tumor cells from initiating metastatic cascade.Sci Rep. 2014 Mar 11;4:4344. doi: 10.1038/srep04344. Sci Rep. 2014. PMID: 24614329 Free PMC article.
References
-
- Fried G, Meister B, Râdestad A. Peptide-containing nerves in the human pregnant uterine cervix: an immunohistochemical study exploring the effect of RU 486 (mifepristone) Hum Reprod. 1990;5(7):870–876. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

