Partial genetic deletion of neuregulin 1 modulates the effects of stress on sensorimotor gating, dendritic morphology, and HPA axis activity in adolescent mice
- PMID: 24442851
- PMCID: PMC4193694
- DOI: 10.1093/schbul/sbt193
Partial genetic deletion of neuregulin 1 modulates the effects of stress on sensorimotor gating, dendritic morphology, and HPA axis activity in adolescent mice
Abstract
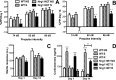
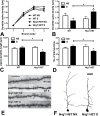
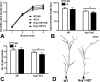
Stress has been linked to the pathogenesis of schizophrenia. Genetic variation in neuregulin 1 (NRG1) increases the risk of developing schizophrenia and may help predict which high-risk individuals will transition to psychosis. NRG1 also modulates sensorimotor gating, a schizophrenia endophenotype. We used an animal model to demonstrate that partial genetic deletion of Nrg1 interacts with stress to promote neurobehavioral deficits of relevance to schizophrenia. Nrg1 heterozygous (HET) mice displayed greater acute stress-induced anxiety-related behavior than wild-type (WT) mice. Repeated stress in adolescence disrupted the normal development of higher prepulse inhibition of startle selectively in Nrg1 HET mice but not in WT mice. Further, repeated stress increased dendritic spine density in pyramidal neurons of the medial prefrontal cortex (mPFC) selectively in Nrg1 HET mice. Partial genetic deletion of Nrg1 also modulated the adaptive response of the hypothalamic-pituitary-adrenal axis to repeated stress, with Nrg1 HET displaying a reduced repeated stress-induced level of plasma corticosterone than WT mice. Our results demonstrate that Nrg1 confers vulnerability to repeated stress-induced sensorimotor gating deficits, dendritic spine growth in the mPFC, and an abberant endocrine response in adolescence.
Keywords: PPI; adolescence; dendritic morphology; neuregulin 1; schizophrenia; stress.
© The Author 2014. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Behavioural effects of high fat diet exposure starting in late adolescence in neuregulin 1 transmembrane domain mutant mice.Behav Brain Res. 2019 Nov 5;373:112074. doi: 10.1016/j.bbr.2019.112074. Epub 2019 Jul 5. Behav Brain Res. 2019. PMID: 31284014
-
Adolescent neuregulin 1 heterozygous mice display enhanced behavioural sensitivity to methamphetamine.Prog Neuropsychopharmacol Biol Psychiatry. 2012 Dec 3;39(2):376-81. doi: 10.1016/j.pnpbp.2012.07.014. Epub 2012 Jul 29. Prog Neuropsychopharmacol Biol Psychiatry. 2012. PMID: 22850204
-
Partial genetic deletion of neuregulin 1 and adolescent stress interact to alter NMDA receptor binding in the medial prefrontal cortex.Front Behav Neurosci. 2014 Sep 29;8:298. doi: 10.3389/fnbeh.2014.00298. eCollection 2014. Front Behav Neurosci. 2014. PMID: 25324742 Free PMC article.
-
[Stress and psychotic transition: A literature review].Encephale. 2016 Aug;42(4):367-73. doi: 10.1016/j.encep.2015.10.001. Epub 2016 May 6. Encephale. 2016. PMID: 27161263 Review. French.
-
Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest.Brain Res. 2009 Oct 13;1293:108-13. doi: 10.1016/j.brainres.2009.03.062. Epub 2009 Apr 8. Brain Res. 2009. PMID: 19361488 Free PMC article. Review.
Cited by
-
Disrupted hippocampal neuregulin-1/ErbB3 signaling and dentate gyrus granule cell alterations in suicide.Transl Psychiatry. 2017 Jul 4;7(7):e1161. doi: 10.1038/tp.2017.132. Transl Psychiatry. 2017. PMID: 28675388 Free PMC article.
-
Animal models of gene-environment interaction in schizophrenia: A dimensional perspective.Prog Neurobiol. 2016 Jan;136:1-27. doi: 10.1016/j.pneurobio.2015.10.002. Epub 2015 Oct 25. Prog Neurobiol. 2016. PMID: 26510407 Free PMC article. Review.
-
Schizophrenia: a consequence of gene-environment interactions?Front Behav Neurosci. 2014 Dec 23;8:435. doi: 10.3389/fnbeh.2014.00435. eCollection 2014. Front Behav Neurosci. 2014. PMID: 25566003 Free PMC article. No abstract available.
-
Specialized Information Processing Deficits and Distinct Metabolomic Profiles Following TM-Domain Disruption of Nrg1.Schizophr Bull. 2017 Sep 1;43(5):1100-1113. doi: 10.1093/schbul/sbw189. Schizophr Bull. 2017. PMID: 28338897 Free PMC article.
-
Neuregulin 1 Deficiency Modulates Adolescent Stress-Induced Dendritic Spine Loss in a Brain Region-Specific Manner and Increases Complement 4 Expression in the Hippocampus.Schizophr Bull. 2019 Mar 7;45(2):339-349. doi: 10.1093/schbul/sby029. Schizophr Bull. 2019. PMID: 29566220 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

