Helminth therapy (worms) for induction of remission in inflammatory bowel disease
- PMID: 24442917
- PMCID: PMC10767620
- DOI: 10.1002/14651858.CD009400.pub2
Helminth therapy (worms) for induction of remission in inflammatory bowel disease
Abstract
Background: Inflammatory bowel disease (IBD) is a chronic, globally-occurring gastrointestinal disorder and a major cause of illness and disability. It is conventionally classified into Crohn's disease (CD) and ulcerative colitis (UC). Helminths are parasitic worms with complex life cycles involving tissue- or lumen-dwelling stages in their hosts, and causing long-lasting or chronic infections that are frequently asymptomatic. Helminths modulate immune responses of their hosts, and many observational and experimental studies support the hypothesis that helminths suppress immune-mediated chronic inflammation that occurs in asthma, allergy and IBD.
Objectives: The objective was to evaluate the efficacy and safety of helminth treatment for induction of remission in IBD.
Search methods: We searched the following databases from inception to 13 July 2013: MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane Inflammatory Bowel Disease Group Specialized Trials Register. We also searched four online trials registries, and abstracts from major meetings. There were no language restrictions.
Selection criteria: Randomised controlled trials (RCTs) where the intervention was any helminth species or combination of helminth species, administered in any dose and by any route and for any duration of exposure to people with active CD or UC, confirmed through any combination of clinical, endoscopic and histological criteria were eligible for inclusion.
Data collection and analysis: Two authors independently extracted data and assessed eligibility using a standardized data collection form. We used the RevMan software for analyses. The primary outcome was induction of remission as defined by the included studies. Secondary outcomes included clinical, histologic, or endoscopic improvement as defined by the authors, endoscopic mucosal healing, change in disease activity index score, change in quality of life score, hospital admissions, requirement for intravenous corticosteroids, surgery, study withdrawal and the incidence of adverse events. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for dichotomous outcomes. We calculated the mean difference (MD) and 95% CI for continuous outcomes. We assessed the methodological quality of included studies using the Cochrane risk of bias tool. The overall quality of the evidence supporting each outcome was assessed using the GRADE criteria.
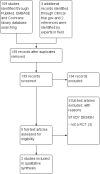
Main results: Two RCTs (90 participants) were included. One trial assessed the efficacy and safety of Trichuris suis (T. suis) ova in patients with UC (n = 54). The other RCT was a phase one that assessed the safety and tolerability of T. suis ova in patients with CD (n = 36). The risk of bias in both studies was judged to be low. In the UC study, during the 12-week study period, participants in the active arm received 2-weekly aliquots of 2500 T. suis eggs, added to 0.8 mL of saline; those in the placebo arm received 0.8 mL saline only. There were sparse data available for the outcomes clinical remission and clinical improvement. Ten per cent (3/30) of patients in the T. suis arm entered remission compared to 4% (1/24) of patients in the placebo arm (RR 2.40, 95% CI 0.27 to 21.63). Forty-three per cent (13/30) of patients in the T. suis group achieved clinical improvement compared to 17% (4/24) of placebo patients (RR 2.60, 95% CI 0.97 to 6.95). The mean ulcerative colitis disease activity index (UCDAI) score was lower in the T. suis group (6.1 +/- 0.61) compared to the placebo group (7.5 +/- 0.66) after 12 weeks of treatment (MD -1.40, 95% CI -1.75 to -1.05). There was only limited evidence relating to the proportion of patients who experienced an adverse event. Three per cent (1/30) of patients in the T. suis group experienced at least one adverse event compared to 12% (3/24) of placebo patients (RR 0.27, 95% CI 0.03 to 2.40). None of the adverse events reported in this study were judged to be related to the study treatment. GRADE analyses rated the overall quality of the evidence for the primary and secondary outcomes (i.e. clinical remission and improvement) as low due to serious imprecision. In the CD study, participants received a single treatment of T. suis ova at a dosage of 500 (n = 9), 2500 (n = 9), or 7500 (n = 9) embryonated eggs or matching placebo (n = 9). The CD study did not assess clinical remission or improvement as outcomes. There were sparse data on adverse events at two weeks. Thirty-seven per cent (10/27) of patients in the T. suis group experienced at least one adverse event compared to 44% (4/9) of placebo patients (RR 0.83, 95% CI 0.35 to 2.01). Only one adverse event (dysgeusia) was judged to be possibly related to treatment in this study. Dysgeusia was reported in one patient in the T. suis group and in one patient in the placebo group.
Authors' conclusions: Currently, there is insufficient evidence to allow any firm conclusions regarding the efficacy and safety of helminths used to treat patients with IBD. The evidence for our primary efficacy outcomes in this review comes from one small study and is of low quality due to serious imprecision. We do not have enough evidence to determine whether helminths are safe when used in patients with UC and CD. Further RCTs are required to assess the efficacy and safety of helminth therapy in IBD.
Conflict of interest statement
PB is a scientific consultant for Coronado Biosciences Inc, a company involved in the development of
The other authors have no financial conflicts of interest and declare that they do not have any association with any manufacturers or promoters of pharmaceutical or helminth products, or with any parties who may have vested interests in the results of this review.
Figures


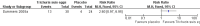
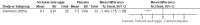





Update of
- doi: 10.1002/14651858.CD009400
Similar articles
-
Azathioprine and 6-mercaptopurine for maintenance of surgically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2014 Aug 1;2014(8):CD010233. doi: 10.1002/14651858.CD010233.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Aug 06;8:CD010233. doi: 10.1002/14651858.CD010233.pub3. PMID: 25081347 Free PMC article. Updated.
-
Aminosalicylates for induction of remission or response in Crohn's disease.Cochrane Database Syst Rev. 2016 Jul 3;7(7):CD008870. doi: 10.1002/14651858.CD008870.pub2. Cochrane Database Syst Rev. 2016. PMID: 27372735 Free PMC article.
-
Etrolizumab for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2015 Dec 2;2015(12):CD011661. doi: 10.1002/14651858.CD011661.pub2. Cochrane Database Syst Rev. 2015. PMID: 26630451 Free PMC article.
-
Oral budesonide for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2015 Oct 26;2015(10):CD007698. doi: 10.1002/14651858.CD007698.pub3. Cochrane Database Syst Rev. 2015. PMID: 26497719 Free PMC article.
-
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease.Cochrane Database Syst Rev. 2015 May 5;(5):CD007572. doi: 10.1002/14651858.CD007572.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2016 Nov 25;11:CD007572. doi: 10.1002/14651858.CD007572.pub3. PMID: 25942580 Updated.
Cited by
-
The status of evolutionary medicine education in North American medical schools.BMC Med Educ. 2015 Mar 8;15:38. doi: 10.1186/s12909-015-0322-5. BMC Med Educ. 2015. PMID: 25884843 Free PMC article.
-
Immunity to gastrointestinal nematode infections.Mucosal Immunol. 2018 Mar;11(2):304-315. doi: 10.1038/mi.2017.113. Epub 2018 Jan 3. Mucosal Immunol. 2018. PMID: 29297502 Review.
-
Helminths in the gastrointestinal tract as modulators of immunity and pathology.Am J Physiol Gastrointest Liver Physiol. 2017 Jun 1;312(6):G537-G549. doi: 10.1152/ajpgi.00024.2017. Epub 2017 Mar 16. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28302598 Free PMC article. Review.
-
Larval Echinococcus multilocularis infection reduces dextran sulphate sodium-induced colitis in mice by attenuating T helper type 1/type 17-mediated immune reactions.Immunology. 2018 May;154(1):76-88. doi: 10.1111/imm.12860. Epub 2017 Dec 8. Immunology. 2018. PMID: 29121394 Free PMC article.
-
The Hygiene Hypothesis and Its Inconvenient Truths about Helminth Infections.PLoS Negl Trop Dis. 2016 Sep 15;10(9):e0004944. doi: 10.1371/journal.pntd.0004944. eCollection 2016 Sep. PLoS Negl Trop Dis. 2016. PMID: 27632204 Free PMC article.
References
References to studies included in this review
Sandborn 2013 {published data only}
-
- Sandborn WJ, Elliott DE, Weinstock J, Summers RW, Landry‐Wheeler A, Silver N, et al. Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn's disease. Alimentary Pharmacology and Therapeutics 2013;38(3):255‐63. - PubMed
Summers 2005a {published data only}
-
- Summers RW, Elliott DE, Urban JF Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 2005;128(4):825‐32. - PubMed
References to studies excluded from this review
Croese 2006 {published data only}
Summers 2003 {published data only}
-
- Summers RW, Elliott DE, Qadir K, Urban JFJ, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. American Journal of Gastroenterology 2003;98(9):2034‐41. - PubMed
References to ongoing studies
NCT01279577 {published data only}
-
- NCT01279577. Trichuris suis ova (TSO) suspension versus placebo in active Crohn's disease (TRUST‐2). http://clinicaltrials.gov/ct2/show/NCT01279577 accessed 13 July 2013.
NCT01434693 {published data only}
-
- NCT01434693. Safety and tolerability of single doses oral CNDO 201 Trichuris suis ova in patients with Crohn's disease. http://clinicaltrials.gov/ct2/show/NCT01434693 accessed 13 July 2013.
Additional references
Akobeng 2003
Bager 2010
-
- Bager P, Arnved J, Rønborg S, Wohlfahrt J, Poulsen LK, Westergaard T, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double‐blind, placebo‐controlled clinical trial. Journal of Allergy and Clinical Immunology 2010;125(1):123‐30. - PubMed
Bager 2011
Behm 2008
Benchimol 2008
Blount 2009
-
- Blount D, Hooi D, Feary J, Venn A, Telford G, Brown A, et al. Immunologic profiles of persons recruited for a randomized, placebo‐controlled clinical trial of hookworm infection. American Journal of Tropical Medicine and Hygiene 2009;81(5):911‐6. - PubMed
Borkow 2000
Brant 1999
-
- Brant R, Sutherland L, Hilsden R. Examining the minimum important difference. Statistics in Medicine 1999;18(19):2593‐603. - PubMed
Chande 2007
Chande 2013
Croft 2012
Daveson 2011
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dwinell 1997
-
- Dwinell MB, Bass P, Schaefer DM, Oaks JA. Tapeworm infection decreases intestinal transit and enteric aerobic bacterial populations. American Journal of Physiology 1997;273(2 Pt 1):G480‐5. - PubMed
Elliott 2000
-
- Elliott DE, Urban JF Jr, Argo CK, Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn's disease?. FASEB Journal 2000;14(12):1848‐55. - PubMed
Elliott 2003
-
- Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF Jr, et al. Exposure to schistosome eggs protects mice from TNBS‐induced colitis. American Journal of Physiology. Gastrointestinal and Liver Physiology 2003;284(3):G385‐91. - PubMed
Elliott 2007
-
- Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune‐mediated inflammation. International Journal for Parasitology 2007;37(5):457‐64. - PubMed
Elliott 2008
Feagan 2012a
Feagan 2012b
Feary 2009
Feary 2010
Finch 2009
-
- Finch RG, Irving WL, Moss P, AndersonJ. Infectious diseases, tropical medicine and sexually transmitted infection. In: Kumar P, Clark M editor(s). Kumar and Clark’s Clinical Medicine. 7th Edition. London: Saunders, 2009.
Hang 2010
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunter 2005
-
- Hunter MM, Wang A, Hirota CL, McKay DM. Neutralizing anti‐IL‐10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. Journal of Immunology 2005;174(11):7368‐75. - PubMed
Ince 2009
Kitagaki 2006
-
- Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. Journal of Immunology 2006;177(3):1628‐35. - PubMed
Lawson 2006
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lim 2010
Mangan 2004
-
- Mangan NE, Fallon RE, Smith P, Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL‐10‐producing B cells. Journal of Immunology 2004;173(10):6346‐56. - PubMed
Marshall 2010
McDonald 2012
McKay 1990
-
- McKay DM, Halton DW, McCaigue MD, Johnston CF, Fairweather I, Shaw C. Hymenolepis diminuta: intestinal goblet cell response to infection in male C57 mice. Experimental Parasitology 1990;71(1):9‐20. - PubMed
Moher 2009
Olano 2006
-
- Olano JP, Weller PF, Guerrant RL, Walker DH. Principles and general considerations. In: Guerrant RL, Walker DH, Weller PF editor(s). Tropical Infectious Diseases – Principles, Pathogens and Practice. 2nd Edition. London: Churchill Livingstone, 2006.
Podolsky 2002
-
- Podolsky DK. Inflammatory bowel disease. New England Journal of Medicine 2002;347(6):417‐29. - PubMed
Prefontaine 2009
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rutgeerts 2005
-
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2005;353(23):2462‐76. - PubMed
Sabin 1996
-
- Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid‐specific Th1‐like immune responses in humans infected with Schistosoma mansoni. Journal of Infectious Diseases 1996;173(1):269‐72. - PubMed
Schnoeller 2008
-
- Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, et al. A helminth immunomodulator reduces allergic and inflammatory response by induction of IL‐10‐producing macrophages. Journal of Immunology 2008;180(6):4265‐72. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Seow 2008
Smith 2007
-
- Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, Rooijen N, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage‐mediated mechanism. Journal of Immunology 2007;178(7):4557‐66. - PubMed
Strickland 1999
-
- Strickland GT. Helminthic infections. In: Strickland GT editor(s). Hunter's Tropical Medicine and Emerging Infectious Diseases. 9th Edition. Philadelphia, Pa: Saunders, 1999.
Timmer 2012
Wammes 2010
-
- Wammes LJ, Hamid F, Wiria AE, Gier B, Sartono E, Maizels RM, et al. Regulatory T cells in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. European Journal of Immunology 2010;40(2):437‐42. - PubMed
Wang 2012
Weller 2008
-
- Weller PF, Nutman TB. Intestinal nematodes. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al. editor(s). Harrison’s Principles of Internal Medicine. 17th Edition. New York: McGraw‐Hill, 2008.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

