Concise review: parthenote stem cells for regenerative medicine: genetic, epigenetic, and developmental features
- PMID: 24443005
- PMCID: PMC3952929
- DOI: 10.5966/sctm.2013-0127
Concise review: parthenote stem cells for regenerative medicine: genetic, epigenetic, and developmental features
Abstract
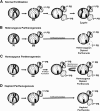
Embryonic stem cells (ESCs) have the potential to provide unlimited cells and tissues for regenerative medicine. ESCs derived from fertilized embryos, however, will most likely be rejected by a patient's immune system unless appropriately immunomatched. Pluripotent stem cells (PSCs) genetically identical to a patient can now be established by reprogramming of somatic cells. However, practical applications of PSCs for personalized therapies are projected to be unfeasible because of the enormous cost and time required to produce clinical-grade cells for each patient. ESCs derived from parthenogenetic embryos (pESCs) that are homozygous for human leukocyte antigens may serve as an attractive alternative for immunomatched therapies for a large population of patients. In this study, we describe the biology and genetic nature of mammalian parthenogenesis and review potential advantages and limitations of pESCs for cell-based therapies.
Keywords: Histocompatibility; Imprinting; Parthenogenesis; Pluripotent stem cells.
Figures
Similar articles
-
Birth of parthenote mice directly from parthenogenetic embryonic stem cells.Stem Cells. 2009 Sep;27(9):2136-45. doi: 10.1002/stem.158. Stem Cells. 2009. PMID: 19544532
-
[Histocompatibility and imprinting status of parthenogenetic embryonic stem cells].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010 Oct;27(5):1158-61. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010. PMID: 21089690 Review. Chinese.
-
Insulin-like growth factor 2 promotes osteogenic cell differentiation in the parthenogenetic murine embryonic stem cells.Tissue Eng Part A. 2012 Feb;18(3-4):331-41. doi: 10.1089/ten.TEA.2011.0074. Epub 2011 Oct 18. Tissue Eng Part A. 2012. PMID: 21902466
-
Human parthenogenetic embryonic stem cells: one potential resource for cell therapy.Sci China C Life Sci. 2009 Jul;52(7):599-602. doi: 10.1007/s11427-009-0096-2. Epub 2009 Jul 30. Sci China C Life Sci. 2009. PMID: 19641863 Review.
-
Discovery of a novel imprinted gene by transcriptional analysis of parthenogenetic embryonic stem cells.Hum Reprod. 2010 Aug;25(8):1927-41. doi: 10.1093/humrep/deq144. Epub 2010 Jun 3. Hum Reprod. 2010. PMID: 20522441 Free PMC article.
Cited by
-
Mitochondrial replacement therapy and assisted reproductive technology: A paradigm shift toward treatment of genetic diseases in gametes or in early embryos.Reprod Med Biol. 2018 Sep 19;17(4):421-433. doi: 10.1002/rmb2.12230. eCollection 2018 Oct. Reprod Med Biol. 2018. PMID: 30377395 Free PMC article. Review.
-
Human Parthenogenetic Embryonic Stem Cell-Derived Neural Stem Cells Express HLA-G and Show Unique Resistance to NK Cell-Mediated Killing.Mol Med. 2015 Mar 23;21(1):185-96. doi: 10.2119/molmed.2014.00188. Mol Med. 2015. PMID: 25811991 Free PMC article.
-
Human pluripotent stem cells: Prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell-based cardiac repair.Adv Drug Deliv Rev. 2016 Jan 15;96:3-17. doi: 10.1016/j.addr.2015.05.004. Epub 2015 May 14. Adv Drug Deliv Rev. 2016. PMID: 25980938 Free PMC article. Review.
-
Neural Stem Cell Tumorigenicity and Biodistribution Assessment for Phase I Clinical Trial in Parkinson's Disease.Sci Rep. 2016 Sep 30;6:34478. doi: 10.1038/srep34478. Sci Rep. 2016. PMID: 27686862 Free PMC article.
-
Myocardial Tissue Engineering for Regenerative Applications.Curr Cardiol Rep. 2017 Sep;19(9):78. doi: 10.1007/s11886-017-0892-4. Curr Cardiol Rep. 2017. PMID: 28752277 Review.
References
-
- Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet. 2012;379:713–720. - PubMed
-
- Advanced Cell Technology. Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With Stargardt's Macular Dystrophy (SMD). Available at http://clinicaltrials.gov/show/NCT01469832 Accessed July 8, 2013.
-
- Drukker M, Benvenisty N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 2004;22:136–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

