Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the Clinical Outcome Assessment in Surgical Trials assessment tool
- PMID: 24443006
- PMCID: PMC3925048
- DOI: 10.5966/sctm.2013-0025
Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the Clinical Outcome Assessment in Surgical Trials assessment tool
Abstract
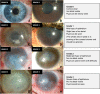
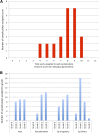
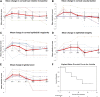
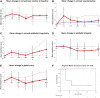
Limbal stem cell deficiency (LSCD) is an eye disorder in which the stem cells responsible for forming the surface skin of the cornea are destroyed by disease. This results in pain, loss of vision, and a cosmetically unpleasant appearance. Many new treatments, including stem cell therapies, are emerging for the treatment of this condition, but assessment of these new technologies is severely hampered by the lack of biomarkers for this disease or validated tools for assessing its severity. The aims of this study were to design and test the reliability of a tool for grading LSCD, to define a set of core outcome measures for use in evaluating treatments for this condition, and to demonstrate their utility. This was achieved by using our defined outcome set (which included the Clinical Outcome Assessment in Surgical Trials of Limbal stem cell deficiency [COASTL] tool) to evaluate the 3-year outcomes for allogeneic ex vivo cultivated limbal epithelial transplantation (allo-CLET) in patients who had bilateral total LSCD secondary to aniridia or Stevens-Johnson syndrome. The results demonstrate that our new grading tool for LSCD, the COASTL tool, is reliable and repeatable, and that improvements in the biomarkers used in this tool correlate positively with improvements in visual acuity. The COASTL tool showed that following allo-CLET there was a decrease in LSCD severity and an increase in visual acuity up to 12 months post-treatment, but thereafter LSCD severity and visual acuity progressively deteriorated.
Keywords: Cornea; Outcome measures; Stem cell; Stem cell deficiency; Stem cell transplantation.
Figures

References
-
- Daniels JT, Dart JK, Tuft SJ, et al. Corneal stem cells in review. Wound Repair Regen. 2001;9:483–494. - PubMed
-
- Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646. - PubMed
-
- Kim JY, Djalilian AR, Schwartz GS, et al. Ocular surface reconstruction: Limbal stem cell transplantation. Ophthalmol Clin North Am. 2003;16:67–77. - PubMed
-
- Kinoshita S, Adachi W, Sotozono C, et al. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res. 2001;20:639–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

