Expression and targeting of secreted proteins from Chlamydia trachomatis
- PMID: 24443531
- PMCID: PMC3993338
- DOI: 10.1128/JB.01290-13
Expression and targeting of secreted proteins from Chlamydia trachomatis
Abstract
Chlamydia trachomatis is an obligate intracellular pathogen that replicates in a vacuole termed the inclusion. Many of the interactions of chlamydiae with the host cell are dependent upon bacterial protein synthesis and presumably exposure of these proteins to the cytosol. Because of the dearth of genetic tools for chlamydiae, previous studies examining secreted proteins required the use of heterologous bacterial systems. Recent advances in genetic manipulation of chlamydia now allow for transformation of the bacteria with plasmids. We describe here a shuttle vector system, pBOMB4, that permits expression of recombinant proteins under constitutive or conditional promoter control. We show that the inclusion membrane protein IncD is secreted in a type III-dependent manner from Yersinia pseudotuberculosis and also secreted from C. trachomatis in infected cells where it localizes appropriately to the inclusion membrane. IncD truncated of the first 30 amino acids containing the secretion signal is no longer secreted and is retained by the bacteria. Cytosolic exposure of secreted proteins can be confirmed by using CyaA, GSK, or microinjection assays. A protein predicted to be retained within the bacteria, NrdB is indeed localized to the chlamydia. In addition, we have shown that the chlamydial effector protein, CPAF, which is secreted into the host cell cytosol by a Sec-dependent pathway, also accesses the cytosol when expressed from this system. These assays should prove useful to assess the secretion of other chlamydial proteins that are potentially exposed to the cytosol of the host cell.
Figures


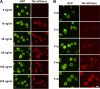

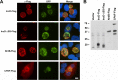
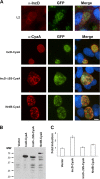


References
-
- Schachter J. 1999. Infection and disease epidemiology, p 139–169 In Stephens RS. (ed), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, DC
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

