Low-frequency nevirapine (NVP)-resistant HIV-1 variants are not associated with failure of antiretroviral therapy in women without prior exposure to single-dose NVP
- PMID: 24443547
- PMCID: PMC3923545
- DOI: 10.1093/infdis/jit635
Low-frequency nevirapine (NVP)-resistant HIV-1 variants are not associated with failure of antiretroviral therapy in women without prior exposure to single-dose NVP
Abstract
Background: Low-frequency nevirapine (NVP)-resistant variants have been associated with virologic failure (VF) of initial NVP-based combination antiretroviral therapy (cART) in women with prior exposure to single-dose NVP (sdNVP). We investigated whether a similar association exists in women without prior sdNVP exposure.
Methods: Pre-cART plasma was analyzed by allele-specific polymerase chain reaction to quantify NVP-resistant mutants in human immunodeficiency virus-infected African women without prior sdNVP who were starting first-line NVP-based cART in the OCTANE/A5208 trial 2. Associations between NVP-resistant mutants and VF or death were determined and compared with published results from women participating in the OCTANE/A5208 trial 1 who had taken sdNVP and initiated NVP-based cART.
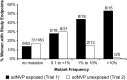
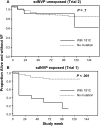
Results: Pre-cART NVP-resistant variants were detected in 18% (39/219) of women without prior sdNVP exposure, compared to 45% (51/114) with prior sdNVP exposure (P < .001). Among women without prior sdNVP exposure, 8 of 39 (21%) with NVP-resistant variants experienced VF or death vs 31 of 180 (17%) without such variants (P = .65); this compares with 21 of 51 (41%) vs 9 of 63 (14%) among women with prior exposure (P = .001).
Conclusions: The risk of VF on NVP-based cART from NVP-resistant variants differs between sdNVP-exposed and -unexposed women. This difference may be driven by drug-resistance mutations emerging after sdNVP exposure that are linked on the same viral genome.
Clinical trials registration: NCT00089505.
Keywords: antiretroviral therapy failure; minor drug-resistant variants; single-dose nevirapine.
Figures


Comment in
-
HIV-1 drug-resistant minority variants: sweating the small stuff.J Infect Dis. 2014 Mar 1;209(5):639-41. doi: 10.1093/infdis/jit656. Epub 2013 Nov 23. J Infect Dis. 2014. PMID: 24273184 Free PMC article. No abstract available.
References
-
- Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069456/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- 1 U01-AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- 1U01 AI069494-01/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- 1 U01AI068636-01/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

