A general approach to site-specific antibody drug conjugates
- PMID: 24443552
- PMCID: PMC3918752
- DOI: 10.1073/pnas.1321237111
A general approach to site-specific antibody drug conjugates
Abstract
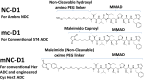
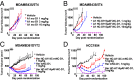
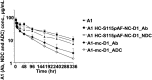
Using an expanded genetic code, antibodies with site-specifically incorporated nonnative amino acids were produced in stable cell lines derived from a CHO cell line with titers over 1 g/L. Using anti-5T4 and anti-Her2 antibodies as model systems, site-specific antibody drug conjugates (NDCs) were produced, via oxime bond formation between ketones on the side chain of the incorporated nonnative amino acid and hydroxylamine functionalized monomethyl auristatin D with either protease-cleavable or noncleavable linkers. When noncleavable linkers were used, these conjugates were highly stable and displayed improved in vitro efficacy as well as in vivo efficacy and pharmacokinetic stability in rodent models relative to conventional antibody drug conjugates conjugated through either engineered surface-exposed or reduced interchain disulfide bond cysteine residues. The advantages of the oxime-bonded, site-specific NDCs were even more apparent when low-antigen-expressing (2+) target cell lines were used in the comparative studies. NDCs generated with protease-cleavable linkers demonstrated that the site of conjugation had a significant impact on the stability of these rationally designed prodrug linkers. In a single-dose rat toxicology study, a site-specific anti-Her2 NDC was well tolerated at dose levels up to 90 mg/kg. These experiments support the notion that chemically defined antibody conjugates can be synthesized in commercially relevant yields and can lead to antibody drug conjugates with improved properties relative to the heterogeneous conjugates formed by nonspecific chemical modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Antibody engineering: fine-tuning antibody-drug conjugates.Nat Rev Drug Discov. 2014 Mar;13(3):178. doi: 10.1038/nrd4266. Nat Rev Drug Discov. 2014. PMID: 24577396 No abstract available.
References
-
- Sapra P, Hooper AT, O’Donnell CJ, Gerber H-P. Investigational antibody drug conjugates for solid tumors. Expert Opin Investig Drugs. 2011;20(8):1131–1149. - PubMed
-
- Wu AM, Senter PD. Arming antibodies: Prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23(9):1137–1146. - PubMed
-
- Polakis P. Arming antibodies for cancer therapy. Curr Opin Pharmacol. 2005;5(4):382–387. - PubMed
-
- Carter PJ, Senter PD. Antibody-drug conjugates for cancer therapy. Cancer J. 2008;14(3):154–169. - PubMed
-
- Hamblett KJ, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–7070. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

