Follistatin promotes adipocyte differentiation, browning, and energy metabolism
- PMID: 24443561
- PMCID: PMC3934723
- DOI: 10.1194/jlr.M039719
Follistatin promotes adipocyte differentiation, browning, and energy metabolism
Abstract
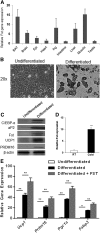
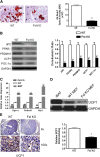
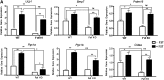
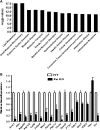
Follistatin (Fst) functions to bind and neutralize the activity of members of the transforming growth factor-β superfamily. Fst has a well-established role in skeletal muscle, but we detected significant Fst expression levels in interscapular brown and subcutaneous white adipose tissue, and further investigated its role in adipocyte biology. Fst expression was induced during adipogenic differentiation of mouse brown preadipocytes and mouse embryonic fibroblasts (MEFs) as well as in cold-induced brown adipose tissue from mice. In differentiated MEFs from Fst KO mice, the induction of brown adipocyte proteins including uncoupling protein 1, PR domain containing 16, and PPAR gamma coactivator-1α was attenuated, but could be rescued by treatment with recombinant FST. Furthermore, Fst enhanced thermogenic gene expression in differentiated mouse brown adipocytes and MEF cultures from both WT and Fst KO groups, suggesting that Fst produced by adipocytes may act in a paracrine manner. Our microarray gene expression profiling of WT and Fst KO MEFs during adipogenic differentiation identified several genes implicated in lipid and energy metabolism that were significantly downregulated in Fst KO MEFs. Furthermore, Fst treatment significantly increases cellular respiration in Fst-deficient cells. Our results implicate a novel role of Fst in the induction of brown adipocyte character and regulation of energy metabolism.
Keywords: brown fat; energy expenditure; mitochondria; mouse embryonic fibroblast; myostatin; uncoupling protein 1.
Figures

References
-
- Haslam D. W., James W. P. 2005. Obesity. Lancet. 366: 1197–1209 - PubMed
-
- Bhatia L. S., Curzen N. P., Byrne C. D. 2012. Nonalcoholic fatty liver disease and vascular risk. Curr. Opin. Cardiol. 27: 420–428 - PubMed
-
- Isidro M. L., Cordido F. 2009. Drug treatment of obesity: established and emerging therapies. Mini Rev. Med. Chem. 9: 664–673 - PubMed
-
- Grundy S. M. 2006. Drug therapy of the metabolic syndrome: minimizing the emerging crisis in polypharmacy. Nat. Rev. Drug Discov. 5: 295–309 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

