The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis
- PMID: 24444773
- PMCID: PMC5525047
- DOI: 10.1016/j.matbio.2014.01.005
The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis
Abstract
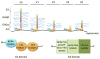
Embryonic development is an exceptionally dynamic process, requiring a provisional extracellular matrix that is amenable to rapid remodeling, and proteolytic or non-proteolytic mechanisms that can remodel the major components of this matrix. Versican is a chondroitin-sulfate proteoglycan that forms highly hydrated complexes with hyaluronan and is widely distributed in the provisional matrix of mammalian embryos. It has been extensively studied in the context of cardiovascular morphogenesis, neural crest cell migration and skeletal development. Analysis of Vcan transgenic mice has established the requirement for versican in cardiac development and its role in skeletogenesis. The ADAMTS family includes several versican-degrading proteases that are active during remodeling of the embryonic provisional matrix, especially during sculpting of versican-rich tissues. Versican is cleaved at specific peptide bonds by ADAMTS proteases, and the cleavage products are detectable by neo-epitope antibodies. Myocardial compaction, closure of the secondary palate (in which neural crest derived cells participate), endocardial cushion remodeling, myogenesis and interdigital web regression are developmental contexts in which ADAMTS-mediated versican proteolysis has been identified as a crucial requirement. ADAMTS proteases are expressed coordinately and function cooperatively in many of these contexts. In addition to versican clearance, ADAMTS proteases generate a bioactive versican fragment containing the N-terminal G1 domain, which we have named versikine. This review promotes the view that the embryonic extracellular matrix has evolved not only to provide a permissive environment for embryo growth and morphogenesis, but through its dissolution to influence and regulate cellular processes.
Keywords: A disintegrin-like and metalloprotease domain with thrombospondin type 1 motifs; ADAMTS; Cardiac jelly; Cleft palate; Embryogenesis; Heart valve; Limb development; Melanoblast; Soft tissue syndactyly; Versican.
Copyright © 2014 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Barascuk N, Genovese F, Larsen L, Byrjalsen I, Zheng Q, Sun S, Hosbond S, Poulsen TS, Diederichsen A, Jensen JM, Mickley H, Register TC, Rasmussen LM, Leeming DJ, Christiansen C, Karsdal MA. A MMP derived versican neoepitope is elevated in plasma from patients with atherosclerotic heart disease. Int J Clin Exp Med. 2013;6:174–184. - PMC - PubMed
-
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

