Selective regulation of axonal growth from developing hippocampal neurons by tumor necrosis factor superfamily member APRIL
- PMID: 24444792
- PMCID: PMC4008386
- DOI: 10.1016/j.mcn.2014.01.002
Selective regulation of axonal growth from developing hippocampal neurons by tumor necrosis factor superfamily member APRIL
Abstract
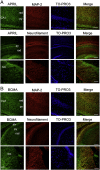
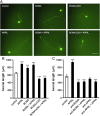
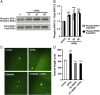
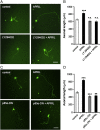
APRIL (A Proliferation-Inducing Ligand, TNFSF13) is a member of the tumor necrosis factor superfamily that regulates lymphocyte survival and activation and has been implicated in tumorigenesis and autoimmune diseases. Here we report the expression and first known activity of APRIL in the nervous system. APRIL and one of its receptors, BCMA (B-Cell Maturation Antigen, TNFRSF17), are expressed by hippocampal pyramidal cells of fetal and postnatal mice. In culture, these neurons secreted APRIL, and function-blocking antibodies to either APRIL or BCMA reduced axonal elongation. Recombinant APRIL enhanced axonal elongation, but did not influence dendrite elongation. The effect of APRIL on axon elongation was inhibited by anti-BCMA and the expression of a signaling-defective BCMA mutant in these neurons, suggesting that the axon growth-promoting effect of APRIL is mediated by BCMA. APRIL promoted phosphorylation and activation of ERK1, ERK2 and Akt and serine phosphorylation and inactivation of GSK-3β in cultured hippocampal pyramidal cells. Inhibition of MEK1/MEK2 (activators of ERK1/ERK2), PI3-kinase (activator of Akt) or Akt inhibited the axon growth-promoting action of APRIL, as did pharmacological activation of GSK-3β and the expression of a constitutively active form of GSK-3β. These findings suggest that APRIL promotes axon elongation by a mechanism that depends both on ERK signaling and PI3-kinase/Akt/GSK-3β signaling.
Keywords: Axon; Hippocampal pyramidal cell; Tumor necrosis factor receptor superfamily; Tumor necrosis factor superfamily.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Aggarwal B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3:745–756. - PubMed
-
- Alexaki V.I., Notas G., Pelekanou V., Kampa M., Valkanou M., Theodoropoulos P., Stathopoulos E.N., Tsapis A., Castanas E. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors, Fn14, BAFF-R, TACI, and BCMA, at different stages of normal and pathological adipose tissue development. J. Immunol. 2009;183:5948–5956. - PubMed
-
- Atwal J.K., Massie B., Miller F.D., Kaplan D.R. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. - PubMed
-
- Bossen C., Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin. Immunol. 2006;18:263–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

