Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis
- PMID: 24444805
- PMCID: PMC4019391
- DOI: 10.1016/j.neurobiolaging.2013.12.005
Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis
Abstract
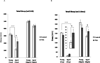
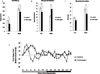
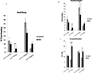
Alterations in the quality, quantity, and architecture of baseline and recovery sleep have been shown to occur during aging. Sleep deprivation induces endoplasmic reticular (ER) stress and upregulates a protective signaling pathway termed the unfolded protein response. The effectiveness of the adaptive unfolded protein response is diminished by age. Previously, we showed that endogenous chaperone levels altered recovery sleep in Drosophila melanogaster. We now report that acute administration of the chemical chaperone sodium 4-phenylbutyrate (PBA) reduces ER stress and ameliorates age-associated sleep changes in Drosophila. PBA consolidates both baseline and recovery sleep in aging flies. The behavioral modifications of PBA are linked to its suppression of ER stress. PBA decreased splicing of X-box binding protein 1 and upregulation of phosphorylated elongation initiation factor 2 α, in flies that were subjected to sleep deprivation. We also demonstrate that directly activating ER stress in young flies fragments baseline sleep and alters recovery sleep. Alleviating prolonged or sustained ER stress during aging contributes to sleep consolidation and improves recovery sleep or sleep debt discharge.
Keywords: 4-phenylbutyrate; Aging; Chaperone; Sleep; Sleep loss/ deprivation; Unfolded protein response.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors state that there are no actual or potential conflicts of interest.
Figures







Similar articles
-
Chemical chaperon 4-phenylbutyrate protects against the endoplasmic reticulum stress-mediated renal fibrosis in vivo and in vitro.Oncotarget. 2016 Apr 19;7(16):22116-27. doi: 10.18632/oncotarget.7904. Oncotarget. 2016. PMID: 26959118 Free PMC article.
-
Selective, potent blockade of the IRE1 and ATF6 pathways by 4-phenylbutyric acid analogues.Br J Pharmacol. 2013 Oct;170(4):822-34. doi: 10.1111/bph.12306. Br J Pharmacol. 2013. PMID: 23869584 Free PMC article.
-
A role for the molecular chaperone protein BiP/GRP78 in Drosophila sleep homeostasis.Sleep. 2007 May;30(5):557-65. doi: 10.1093/sleep/30.5.557. Sleep. 2007. PMID: 17552370
-
[Molecular pharmacological studies on the protection mechanism against endoplasmic reticulum stress-induced neurodegenerative disease].Yakugaku Zasshi. 2012;132(12):1437-42. doi: 10.1248/yakushi.12-00249. Yakugaku Zasshi. 2012. PMID: 23208051 Review. Japanese.
-
The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis.Int J Biochem Cell Biol. 2015 Apr;61:45-52. doi: 10.1016/j.biocel.2015.01.015. Epub 2015 Feb 7. Int J Biochem Cell Biol. 2015. PMID: 25660369 Review.
Cited by
-
Induction of excessive endoplasmic reticulum stress in the Drosophila male accessory gland results in infertility.PLoS One. 2015 Mar 5;10(3):e0119386. doi: 10.1371/journal.pone.0119386. eCollection 2015. PLoS One. 2015. PMID: 25742606 Free PMC article.
-
Sleep, Aging, and Cellular Health: Aged-Related Changes in Sleep and Protein Homeostasis Converge in Neurodegenerative Diseases.Front Aging Neurosci. 2019 Jun 11;11:140. doi: 10.3389/fnagi.2019.00140. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31244649 Free PMC article. Review.
-
Evolutionarily Conserved Regulation of Sleep by the Protein Translational Regulator PERK.Curr Biol. 2020 May 4;30(9):1639-1648.e3. doi: 10.1016/j.cub.2020.02.030. Epub 2020 Mar 12. Curr Biol. 2020. PMID: 32169212 Free PMC article.
-
Understanding the impact of ER stress on lung physiology.Front Cell Dev Biol. 2024 Dec 18;12:1466997. doi: 10.3389/fcell.2024.1466997. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39744015 Free PMC article. Review.
-
Perspective: Modulating the integrated stress response to slow aging and ameliorate age-related pathology.Nat Aging. 2021 Sep;1(9):760-768. doi: 10.1038/s43587-021-00112-9. Epub 2021 Sep 13. Nat Aging. 2021. PMID: 35146440 Free PMC article.
References
-
- Ancoli-Israel S. Sleep problems in older adults: putting myths to bed. Geriatrics. 1997;52:20–30. - PubMed
-
- Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 2005;393:759–772. - PubMed
-
- Empana JP, Dauvilliers Y, Dartigues JF, et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke. 2009;40:1219–1224. - PubMed
-
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

