Angiotensin II induces Fat1 expression/activation and vascular smooth muscle cell migration via Nox1-dependent reactive oxygen species generation
- PMID: 24445059
- PMCID: PMC3943818
- DOI: 10.1016/j.yjmcc.2013.10.013
Angiotensin II induces Fat1 expression/activation and vascular smooth muscle cell migration via Nox1-dependent reactive oxygen species generation
Abstract
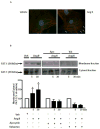
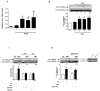
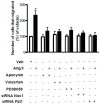
Fat1 is an atypical cadherin that controls vascular smooth muscle cell (VSMC) proliferation and migration. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1 (Nox1) is an important source of reactive oxygen species (ROS) in VSMCs. Angiotensin II (Ang II) induces the expression and/or activation of both Fat1 and Nox1 proteins. This study tested the hypothesis that Ang II-induced Fat1 activation and VSMC migration are mediated by Nox1-dependent ROS generation and redox signaling. Studies were performed in cultured VSMCs from Sprague–Dawley rats. Cells were treated with Ang II (1 μmol/L) for short (5 to 30 min) or long term stimulations (3 to 12 h) in the absence or presence of the antioxidant apocynin (10 μmol/L), extracellular-signal-regulated kinases 1/2 (Erk1/2) inhibitor PD98059 (1 μmol/L), or Ang II type 1 receptor (AT1R) valsartan (1 μmol/L). siRNA was used to knockdown Nox1 or Fat1. Cell migration was determined by Boyden chamber assay. Ang II increased Fat1 mRNA and protein levels and promoted Fat1 translocation to the cell membrane, responses that were inhibited by AT1R antagonist and antioxidant treatment. Downregulation of Nox1 inhibited the effects of Ang II on Fat1 protein expression. Nox1 protein induction, ROS generation, and p44/p42 MAPK phosphorylation in response to Ang II were prevented by valsartan and apocynin, and Nox1 siRNA inhibited Ang II-induced ROS generation. Knockdown of Fat1 did not affect Ang II-mediated increases in Nox1 expression or ROS. Inhibition of p44/p42 MAPK phosphorylation by PD98059 abrogated the Ang II-induced increase in Fat1 expression and membrane translocation. Knockdown of Fat1 inhibited Ang II-induced VSMC migration, which was also prevented by valsartan, apocynin, PD98059, and Nox1 siRNA. Our findings indicate that Ang II regulates Fat1 expression and activity and induces Fat1-dependent VSMC migration via activation of AT1R, ERK1/2, and Nox1-derived ROS, suggesting a role for Fat1 downstream of Ang II signaling that leads to vascular remodeling.
Conflict of interest statement
No potential conflicts of interest are reported.
Figures




Similar articles
-
Angiotensin II and vascular injury.Curr Hypertens Rep. 2014 Jun;16(6):431. doi: 10.1007/s11906-014-0431-2. Curr Hypertens Rep. 2014. PMID: 24760441 Review.
-
The GTPase ARF6 Controls ROS Production to Mediate Angiotensin II-Induced Vascular Smooth Muscle Cell Proliferation.PLoS One. 2016 Jan 29;11(1):e0148097. doi: 10.1371/journal.pone.0148097. eCollection 2016. PLoS One. 2016. PMID: 26824355 Free PMC article.
-
Reactive oxygen species derived from NADPH oxidase 1 and mitochondria mediate angiotensin II-induced smooth muscle cell senescence.J Mol Cell Cardiol. 2016 Sep;98:18-27. doi: 10.1016/j.yjmcc.2016.07.001. Epub 2016 Jul 2. J Mol Cell Cardiol. 2016. PMID: 27381955
-
Antioxidant effect of adrenomedullin on angiotensin II-induced reactive oxygen species generation in vascular smooth muscle cells.Endocrinology. 2004 Jul;145(7):3331-7. doi: 10.1210/en.2003-1583. Epub 2004 Apr 7. Endocrinology. 2004. PMID: 15070851
-
The FAT1 Cadherin Drives Vascular Smooth Muscle Cell Migration.Cells. 2023 Jun 14;12(12):1621. doi: 10.3390/cells12121621. Cells. 2023. PMID: 37371091 Free PMC article. Review.
Cited by
-
PGC-1α limits angiotensin II-induced rat vascular smooth muscle cells proliferation via attenuating NOX1-mediated generation of reactive oxygen species.Biosci Rep. 2015 Aug 26;35(5):e00252. doi: 10.1042/BSR20150112. Biosci Rep. 2015. PMID: 26310573 Free PMC article.
-
The Reciprocal Relationship Between Cell Adhesion Molecules and Reactive Oxygen Species.Cells. 2025 Jul 17;14(14):1098. doi: 10.3390/cells14141098. Cells. 2025. PMID: 40710351 Free PMC article. Review.
-
NADPH Oxidases in Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction.Antioxidants (Basel). 2022 Sep 16;11(9):1822. doi: 10.3390/antiox11091822. Antioxidants (Basel). 2022. PMID: 36139898 Free PMC article. Review.
-
Angiotensin II and vascular injury.Curr Hypertens Rep. 2014 Jun;16(6):431. doi: 10.1007/s11906-014-0431-2. Curr Hypertens Rep. 2014. PMID: 24760441 Review.
-
Apocynin attenuates angiotensin II-induced vascular smooth muscle cells osteogenic switching via suppressing extracellular signal-regulated kinase 1/2.Oncotarget. 2016 Dec 13;7(50):83588-83600. doi: 10.18632/oncotarget.13193. Oncotarget. 2016. PMID: 27835878 Free PMC article.
References
-
- Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, et al. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J. 2003;17(12):1759–61. - PubMed
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. - PubMed
-
- Lemarie CA, Schiffrin EL. The angiotensin II type 2 receptor in cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2010;11:19–31. - PubMed
-
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

