Adult spinal motoneurones are not hyperexcitable in a mouse model of inherited amyotrophic lateral sclerosis
- PMID: 24445319
- PMCID: PMC3979619
- DOI: 10.1113/jphysiol.2013.265843
Adult spinal motoneurones are not hyperexcitable in a mouse model of inherited amyotrophic lateral sclerosis
Abstract
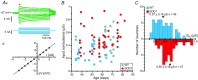
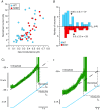
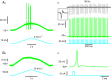
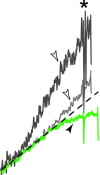
In amyotrophic lateral sclerosis (ALS), an adult onset disease in which there is progressive degeneration of motoneurones, it has been suggested that an intrinsic hyperexcitability of motoneurones (i.e. an increase in their firing rates), contributes to excitotoxicity and to disease onset. Here we show that there is no such intrinsic hyperexcitability in spinal motoneurones. Our studies were carried out in an adult mouse model of ALS with a mutated form of superoxide dismutase 1 around the time of the first muscle fibre denervations. We showed that the recruitment current, the voltage threshold for spiking and the frequency-intensity gain in the primary range are all unchanged in most spinal motoneurones, despite an increased input conductance. On its own, increased input conductance would decrease excitability, but the homeostasis for excitability is maintained due to an upregulation of a depolarizing current that is activated just below the spiking threshold. However, this homeostasis failed in a substantial fraction of motoneurones, which became hypoexcitable and unable to produce sustained firing in response to ramps of current. We found similar results both in lumbar motoneurones recorded in anaesthetized mice, and in sacrocaudal motoneurones recorded in vitro, indicating that the lack of hyperexcitability is not caused by anaesthetics. Our results suggest that, if excitotoxicity is indeed a mechanism leading to degeneration in ALS, it is not caused by the intrinsic electrical properties of motoneurones but by extrinsic factors such as excessive synaptic excitation.
Figures




References
-
- Alves CJ, de Santana LP, dos Santos AJ, de Oliveira GP, Duobles T, Scorisa JM, Martins RS, Maximino JR, Chadi G. Early motor and electrophysiological changes in transgenic mouse model of amyotrophic lateral sclerosis and gender differences on clinical outcome. Brain Res. 2011;1394:90–104. - PubMed
-
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001;86:1955–1971. - PubMed
-
- Bories C, Amendola J, Lamotte d’Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:451–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

