The CD37-targeted antibody-drug conjugate IMGN529 is highly active against human CLL and in a novel CD37 transgenic murine leukemia model
- PMID: 24445867
- PMCID: PMC4090271
- DOI: 10.1038/leu.2014.32
The CD37-targeted antibody-drug conjugate IMGN529 is highly active against human CLL and in a novel CD37 transgenic murine leukemia model
Abstract
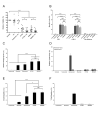
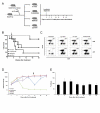
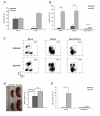
Therapeutic regimens for chronic lymphocytic leukemia (CLL) have increasingly utilized monoclonal antibodies since the chimeric anti-CD20 antibody rituximab was introduced. Despite improved clinical outcomes, current CLL therapies are not curative. Therefore, antibodies with greater efficacy and novel targets are desirable. One promising target is CD37, a tetraspanin protein highly expressed on malignant B-cells in CLL and non-Hodgkin lymphoma. Although several novel CD37-directed therapeutics are emerging, detailed preclinical evaluation of these agents is limited by lack of appropriate animal models with spontaneous leukemia expressing the human CD37 (hCD37) target. To address this, we generated a murine CLL model that develops transplantable hCD37+ leukemia. Subsequently, we engrafted healthy mice with this leukemia to evaluate IMGN529, a novel hCD37-targeting antibody-drug conjugate. IMGN529 rapidly eliminated peripheral blood leukemia and improved overall survival. In contrast, the antibody component of IMGN529 could not alter disease course despite exhibiting substantial in vitro cytotoxicity. Furthermore, IMGN529 is directly cytotoxic to human CLL in vitro, depletes B-cells in patient whole blood and promotes killing by macrophages and natural killer cells. Our results demonstrate the utility of a novel mouse model for evaluating anti-human CD37 therapeutics and highlight the potential of IMGN529 for treatment of CLL and other CD37-positive B-cell malignancies.
Figures



References
-
- Byrd JC, Rai K, Peterson BL, Appelbaum FR, Morrison VA, Kolitz JE, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105(1):49–53. - PubMed
-
- Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63(8):803–43. - PubMed
-
- Woyach JA, Ruppert AS, Heerema NA, Peterson BL, Gribben JG, Morrison VA, et al. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: long-term follow-up of CALGB study 9712. J Clin Oncol. 2011;29(10):1349–55. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

