Roles of Atox1 and p53 in the trafficking of copper-64 to tumor cell nuclei: implications for cancer therapy
- PMID: 24445997
- PMCID: PMC3951176
- DOI: 10.1007/s00775-013-1087-0
Roles of Atox1 and p53 in the trafficking of copper-64 to tumor cell nuclei: implications for cancer therapy
Abstract
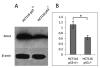
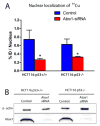
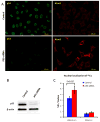
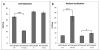
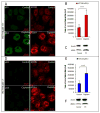
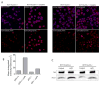
Owing to its cytotoxicity, free copper is chelated by protein side chains and does not exist in vivo. Several chaperones transport copper to various cell compartments, but none have been identified that traffic copper to the nucleus. Copper-64 decays by β (+) and β (-) emission, allowing positron emission tomography and targeted radionuclide therapy for cancer. Because the delivery of (64)Cu to the cell nucleus may enhance the therapeutic effect of copper radiopharmaceuticals, elucidation of the pathway(s) involved in transporting copper to the tumor cell nucleus is important for optimizing treatment. We identified Atox1 as one of the proteins that binds copper in the nucleus. Mouse embryonic fibroblast cells, positive and negative for Atox1, were used to determine the role of Atox1 in (64)Cu transport to the nucleus. Mouse embryonic fibroblast Atox1(+/+) cells accumulated more (64)Cu in the nucleus than did Atox1(-/-) cells. HCT 116 colorectal cancer cells expressing p53 (+/+) and not expressing p53 (-/-) were used to evaluate the role of this tumor suppressor protein in (64)Cu transport. In cells treated with cisplatin, the uptake of (64)Cu in the nucleus of HCT 116 p53(+/+) cells was greater than that in HCT 116 p53(-/-) cells. Atox1 expression increased in HCT 116 p53(+/+) and p53(-/-) cells treated with cisplatin; however, Atox1 localized to the nuclei of p53(+/+) cells more than in the p53(-/-) cells. The data presented here indicate that Atox1 is involved in copper transport to the nucleus, and cisplatin affects nuclear transport of (64)Cu in HCT 116 cells by upregulating the expression and the nuclear localization of Atox1.
Figures

Similar articles
-
The role of p53 in the trafficking of copper-64 to tumor cell nuclei.Cancer Biol Ther. 2008 Jan;7(1):63-9. doi: 10.4161/cbt.7.1.5130. Epub 2007 Oct 8. Cancer Biol Ther. 2008. PMID: 17938576
-
Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation.J Biol Chem. 2008 Apr 4;283(14):9157-67. doi: 10.1074/jbc.M709463200. Epub 2008 Feb 2. J Biol Chem. 2008. PMID: 18245776 Free PMC article.
-
Cisplatin binds human copper chaperone Atox1 and promotes unfolding in vitro.Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):6951-6. doi: 10.1073/pnas.1012899108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482801 Free PMC article.
-
An expanding range of functions for the copper chaperone/antioxidant protein Atox1.Antioxid Redox Signal. 2013 Sep 20;19(9):945-57. doi: 10.1089/ars.2012.5086. Epub 2013 Feb 6. Antioxid Redox Signal. 2013. PMID: 23249252 Free PMC article. Review.
-
Copper chaperone antioxidant 1: multiple roles and a potential therapeutic target.J Mol Med (Berl). 2023 May;101(5):527-542. doi: 10.1007/s00109-023-02311-w. Epub 2023 Apr 5. J Mol Med (Berl). 2023. PMID: 37017692 Review.
Cited by
-
Identification of New Potential Interaction Partners for Human Cytoplasmic Copper Chaperone Atox1: Roles in Gene Regulation?Int J Mol Sci. 2015 Jul 23;16(8):16728-39. doi: 10.3390/ijms160816728. Int J Mol Sci. 2015. PMID: 26213915 Free PMC article.
-
Identification and validation of a novel cuproptosis-related genes signature associated with prognosis, clinical implications and immunotherapy of hepatocellular carcinoma.Front Pharmacol. 2023 Feb 9;14:1088993. doi: 10.3389/fphar.2023.1088993. eCollection 2023. Front Pharmacol. 2023. PMID: 36843949 Free PMC article.
-
Silver Ions as a Tool for Understanding Different Aspects of Copper Metabolism.Nutrients. 2019 Jun 17;11(6):1364. doi: 10.3390/nu11061364. Nutrients. 2019. PMID: 31213024 Free PMC article. Review.
-
Regulation of anti-tumor immunity by metal ion in the tumor microenvironment.Front Immunol. 2024 Jun 10;15:1379365. doi: 10.3389/fimmu.2024.1379365. eCollection 2024. Front Immunol. 2024. PMID: 38915413 Free PMC article. Review.
-
Copper metabolism in hepatocellular carcinoma: from molecular mechanisms to therapeutic opportunities.Front Mol Biosci. 2025 May 13;12:1578693. doi: 10.3389/fmolb.2025.1578693. eCollection 2025. Front Mol Biosci. 2025. PMID: 40433591 Free PMC article. Review.
References
-
- Puig S, Thiele DJ. Current opinion in chemical biology. 2002;6:171–180. - PubMed
-
- Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Science. 1999;284:805–808. - PubMed
-
- Lewis MR, Wang M, Axworthy DB, Theodore LJ, Mallet RW, Fritzberg AR, Welch MJ, Anderson CJ. Journal of nuclear medicine : official publication. Society of Nuclear Medicine. 2003;44:1284–1292. - PubMed
-
- Anderson CJ, Pajeau TS, Edwards WB, Sherman EL, Rogers BE, Welch MJ. Journal of nuclear medicine : official publication. Society of Nuclear Medicine. 1995;36:2315–2325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

