Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation
- PMID: 24446074
- PMCID: PMC4105336
- DOI: 10.1007/s00429-014-0709-9
Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation
Abstract
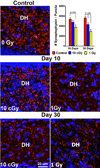
Cranial radiotherapy is used routinely to control the growth of primary and secondary brain tumors, but often results in serious and debilitating cognitive dysfunction. In part due to the beneficial dose depth distributions that may spare normal tissue damage, the use of protons to treat CNS and other tumor types is rapidly gaining popularity. Astronauts exposed to lower doses of protons in the space radiation environment are also at risk for developing adverse CNS complications. To explore the consequences of whole body proton irradiation, mice were subjected to 0.1 and 1 Gy and analyzed for morphometric changes in hippocampal neurons 10 and 30 days following exposure. Significant dose-dependent reductions (~33 %) in dendritic complexity were found, when dendritic length, branching and area were analyzed 30 days after exposure. At equivalent doses and times, significant reductions in the number (~30 %) and density (50-75 %) of dendritic spines along hippocampal neurons of the dentate gyrus were also observed. Immature spines (filopodia, long) exhibited the greatest sensitivity (1.5- to 3-fold) to irradiation, while more mature spines (mushroom) were more resistant to changes over a 1-month post-irradiation timeframe. Irradiated granule cell neurons spanning the subfields of the dentate gyrus showed significant and dose-responsive reductions in synaptophysin expression, while the expression of postsynaptic density protein (PSD-95) was increased significantly. These findings corroborate our past work using photon irradiation, and demonstrate for the first time, dose-responsive changes in dendritic complexity, spine density and morphology and synaptic protein levels following exposure to low-dose whole body proton irradiation.
Conflict of interest statement
The authors declare no conflicts of interest
Figures




References
-
- Armstrong DD, Dunn K, Antalffy B. Decreased dendritic branching in frontal, motor and limbic cortex in Rett syndrome compared with trisomy 21. Journal of neuropathology and experimental neurology. 1998;57(11):1013–1017. - PubMed
-
- Barani IJ, Cuttino LW, Benedict SH, Todor D, Bump EA, Wu Y, Chung TD, Broaddus WC, Lin PS. Neural stem cell-preserving external-beam radiotherapy of central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2007;68(4):978–985. - PubMed
-
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17(3):381–386. - PubMed
-
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Functional neuroanatomical correlates of the effects of stress on memory. Journal of traumatic stress. 1995;8(4):527–553. - PubMed
-
- Britten RA, Davis LK, Johnson AM, Keeney S, Siegel A, Sanford LD, Singletary SJ, Lonart G. Low (20 cGy) doses of 1 GeV/u (56)Fe--particle radiation lead to a persistent reduction in the spatial learning ability of rats. Radiat Res. 2012;177(2):146–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

