Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension
- PMID: 24446489
- PMCID: PMC3920564
- DOI: 10.1084/jem.20111741
Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension
Abstract
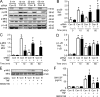
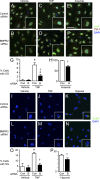
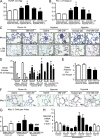
Idiopathic pulmonary arterial hypertension (PAH [IPAH]) is an insidious and potentially fatal disease linked to a mutation or reduced expression of bone morphogenetic protein receptor 2 (BMPR2). Because intravascular inflammatory cells are recruited in IPAH pathogenesis, we hypothesized that reduced BMPR2 enhances production of the potent chemokine granulocyte macrophage colony-stimulating factor (GM-CSF) in response to an inflammatory perturbation. When human pulmonary artery (PA) endothelial cells deficient in BMPR2 were stimulated with tumor necrosis factor (TNF), a twofold increase in GM-CSF was observed and related to enhanced messenger RNA (mRNA) translation. The mechanism was associated with disruption of stress granule formation. Specifically, loss of BMPR2 induced prolonged phospho-p38 mitogen-activated protein kinase (MAPK) in response to TNF, and this increased GADD34-PP1 phosphatase activity, dephosphorylating eukaryotic translation initiation factor (eIF2α), and derepressing GM-CSF mRNA translation. Lungs from IPAH patients versus unused donor controls revealed heightened PA expression of GM-CSF co-distributing with increased TNF and expanded populations of hematopoietic and endothelial GM-CSF receptor α (GM-CSFRα)-positive cells. Moreover, a 3-wk infusion of GM-CSF in mice increased hypoxia-induced PAH, in association with increased perivascular macrophages and muscularized distal arteries, whereas blockade of GM-CSF repressed these features. Thus, reduced BMPR2 can subvert a stress granule response, heighten GM-CSF mRNA translation, increase inflammatory cell recruitment, and exacerbate PAH.
Figures





References
-
- Alastalo T.P., Li M., Perez V.J., Pham D., Sawada H., Wang J.K., Koskenvuo M., Wang L., Freeman B.A., Chang H.Y., Rabinovitch M. 2011. Disruption of PPARγ/β-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J. Clin. Invest. 121:3735–3746 10.1172/JCI43382 - DOI - PMC - PubMed
-
- Atkinson C., Stewart S., Upton P.D., Machado R., Thomson J.R., Trembath R.C., Morrell N.W. 2002. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 105:1672–1678 10.1161/01.CIR.0000012754.72951.3D - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

