Fungal endophthalmitis associated with compounded products
- PMID: 24447640
- PMCID: PMC3901475
- DOI: 10.3201/eid2002.131257
Fungal endophthalmitis associated with compounded products
Abstract
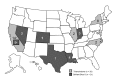
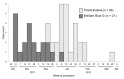
Fungal endophthalmitis is a rare but serious infection. In March 2012, several cases of probable and laboratory-confirmed fungal endophthalmitis occurring after invasive ocular procedures were reported nationwide. We identified 47 cases in 9 states: 21 patients had been exposed to the intraocular dye Brilliant Blue G (BBG) during retinal surgery, and the other 26 had received an intravitreal injection containing triamcinolone acetonide. Both drugs were produced by Franck's Compounding Lab (Ocala, FL, USA). Fusarium incarnatum-equiseti species complex mold was identified in specimens from BBG-exposed case-patients and an unopened BBG vial. Bipolaris hawaiiensis mold was identified in specimens from triamcinolone-exposed case-patients. Exposure to either product was the only factor associated with case status. Of 40 case-patients for whom data were available, 39 (98%) lost vision. These concurrent outbreaks, associated with 1 compounding pharmacy, resulted in a product recall. Ensuring safety and integrity of compounded medications is critical for preventing further outbreaks associated with compounded products.
Keywords: Bipolaris hawaiiensis; Franck’s; Fusarium incarnatum-equiseti; compounding; disease outbreaks; endophthalmitis; eye infections; fungal; fungi; mold.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

