Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice
- PMID: 24447795
- PMCID: PMC3907495
- DOI: 10.1186/1472-6882-14-37
Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice
Abstract
Background: Alzheimer's disease (AD) is a severe neurodegenerative disease for which there is currently no effective treatment. The purpose of this study was to investigate whether repeated electroacupuncture (EA) stimulation would improve cognitive function and the pathological features of AD in amyloid precursor protein (APP)/presenilin 1 (PS1) double transgenic mice.
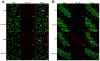
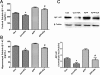
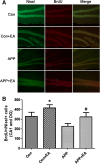
Methods: Cognitive function of APP/PS1 double transgenic mice was assessed using the Morris water maze test before and after EA treatment. Levels of amyloid β-peptide (Aβ) deposits in the hippocampus and cortex were evaluated by immunofluorescence, western blot and enzyme-linked immunosorbent assay. Expression of brain-derived neurotrophic factor (BDNF) was also examined by immunofluorescence and western blot. The neurogenesis was labeled by the DNA marker bromodeoxyuridine.
Results: EA stimulation significantly ameliorated the learning and memory deficits of AD mice by shortening escape latency and increasing the time spent in the target zone during the probe test. Additionally, decreased Aβ deposits and increased BDNF expression and neurogenesis in the hippocampus and cortex of EA-treated AD mice were detected. The same change was detected in wild-type mice after EA treatment compared with wild-type mice without EA treatment.
Conclusions: Repeated EA stimulation may improve cognitive function, attenuate Aβ deposits, up-regulate the expression of BDNF and promote neurogenesis in the APP/PS1 double transgenic mice. This suggests that EA may be a promising treatment for AD.
Figures



Similar articles
-
Electroacupuncture attenuates reference memory impairment associated with astrocytic NDRG2 suppression in APP/PS1 transgenic mice.Mol Neurobiol. 2014 Oct;50(2):305-13. doi: 10.1007/s12035-013-8609-1. Epub 2014 Jan 5. Mol Neurobiol. 2014. PMID: 24390566
-
Electroacupuncture Alleviates Memory Deficits in APP/PS1 Mice by Targeting Serotonergic Neurons in Dorsal Raphe Nucleus.Curr Med Sci. 2024 Oct;44(5):987-1000. doi: 10.1007/s11596-024-2908-9. Epub 2024 Jul 11. Curr Med Sci. 2024. PMID: 38990450
-
Electroacupuncture activates AMPKα1 to improve learning and memory in the APP/PS1 mouse model of early Alzheimer's disease by regulating hippocampal mitochondrial dynamics.J Integr Med. 2024 Sep;22(5):588-599. doi: 10.1016/j.joim.2024.08.002. Epub 2024 Aug 9. J Integr Med. 2024. PMID: 39181774
-
When neurogenesis encounters aging and disease.Trends Neurosci. 2010 Dec;33(12):569-79. doi: 10.1016/j.tins.2010.09.003. Epub 2010 Oct 18. Trends Neurosci. 2010. PMID: 20961627 Free PMC article. Review.
-
Mechanisms of the Beneficial Effects of Exercise on Brain-Derived Neurotrophic Factor Expression in Alzheimer's Disease.Biomolecules. 2023 Oct 26;13(11):1577. doi: 10.3390/biom13111577. Biomolecules. 2023. PMID: 38002258 Free PMC article. Review.
Cited by
-
A review on traditional Chinese medicine natural products and acupuncture intervention for Alzheimer's disease based on the neuroinflammatory.Chin Med. 2024 Feb 28;19(1):35. doi: 10.1186/s13020-024-00900-6. Chin Med. 2024. PMID: 38419106 Free PMC article. Review.
-
Potential benefits of mesenchymal stem cells and electroacupuncture on the trophic factors associated with neurogenesis in mice with ischemic stroke.Sci Rep. 2018 Feb 1;8(1):2044. doi: 10.1038/s41598-018-20481-3. Sci Rep. 2018. PMID: 29391466 Free PMC article.
-
Effect of Combined Electroacupuncture and Selegiline Treatment in Alzheimer's Disease: An Animal Model.Front Pharmacol. 2020 Dec 10;11:606480. doi: 10.3389/fphar.2020.606480. eCollection 2020. Front Pharmacol. 2020. PMID: 33362561 Free PMC article.
-
Electroacupuncture improves cognitive deficits associated with AMPK activation in SAMP8 mice.Metab Brain Dis. 2015 Jun;30(3):777-84. doi: 10.1007/s11011-014-9641-1. Epub 2014 Dec 12. Metab Brain Dis. 2015. PMID: 25502012
-
Neuroprotective Effects of Electroacupuncture on an Animal Model of Bilateral Common Carotid Artery Occlusion.Mol Neurobiol. 2016 Dec;53(10):7228-7236. doi: 10.1007/s12035-015-9610-7. Epub 2015 Dec 21. Mol Neurobiol. 2016. PMID: 26687230
References
-
- Hampel H, Prvulovic D, Teipel S, Jessen F, Luckhaus C, Frölich L, Riepe MW, Dodel R, Leyhe T, Bertram L, Hoffmann W, Faltraco F. German Task Force on Alzheimer’s Disease (GTF-AD) The future of Alzheimer’s disease: the next 10 years. Prog Neurobiol. 2011;95:718–728. doi: 10.1016/j.pneurobio.2011.11.008. - DOI - PubMed
-
- Selkoe DJ. Images in neuroscience. Alzheimer’s disease: from genes to pathogenesis. Am J Psychiatry. 1997;154:1198. - PubMed
-
- Chuang TT. Neurogenesis in mouse models of Alzheimer’s disease. Biochim Biophys Acta. 1802;2010:872–880. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

