A role for the pattern recognition receptor Nod2 in promoting recruitment of CD103+ dendritic cells to the colon in response to Trichuris muris infection
- PMID: 24448097
- PMCID: PMC4074062
- DOI: 10.1038/mi.2013.125
A role for the pattern recognition receptor Nod2 in promoting recruitment of CD103+ dendritic cells to the colon in response to Trichuris muris infection
Abstract
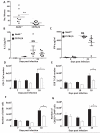
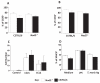
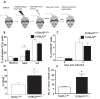
The ability of the colon to generate an immune response to pathogens, such as the model pathogen Trichuris muris, is a fundamental and critical defense mechanism. Resistance to T. muris infection is associated with the rapid recruitment of dendritic cells (DCs) to the colonic epithelium via epithelial chemokine production. However, the epithelial-pathogen interactions that drive chemokine production are not known. We addressed the role of the cytosolic pattern recognition receptor Nod2. In response to infection, there was a rapid influx of CD103(+)CD11c(+) DCs into the colonic epithelium in wild-type (WT) mice, whereas this was absent in Nod2(-/-) animals. In vitro chemotaxis assays and in vivo experiments using bone marrow chimeras of WT mice reconstituted with Nod2(-/-) bone marrow and infected with T. muris demonstrated that the migratory function of Nod2(-/-) DCs was normal. Investigation of colonic epithelial cell (CEC) innate responses revealed a significant reduction in epithelial production of the chemokines CCL2 and CCL5 but not CCL20 by Nod2-deficient CECs. Collectively, these data demonstrate the importance of Nod2 in CEC responses to infection and the requirement for functional Nod2 in initiating host epithelial chemokine-mediated responses and subsequent DC recruitment and T-cell responses following infection.
Figures




Similar articles
-
Rapid dendritic cell mobilization to the large intestinal epithelium is associated with resistance to Trichuris muris infection.J Immunol. 2009 Mar 1;182(5):3055-62. doi: 10.4049/jimmunol.0802749. J Immunol. 2009. PMID: 19234202 Free PMC article.
-
NOD2 modulates immune tolerance via the GM-CSF-dependent generation of CD103+ dendritic cells.Proc Natl Acad Sci U S A. 2020 May 19;117(20):10946-10957. doi: 10.1073/pnas.1912866117. Epub 2020 Apr 29. Proc Natl Acad Sci U S A. 2020. PMID: 32350141 Free PMC article.
-
Assessing the role of CD103 in immunity to an intestinal helminth parasite.PLoS One. 2011 May 5;6(5):e19580. doi: 10.1371/journal.pone.0019580. PLoS One. 2011. PMID: 21573179 Free PMC article.
-
Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103(+) dendritic cells.Mucosal Immunol. 2016 Jul;9(4):894-906. doi: 10.1038/mi.2015.118. Epub 2015 Nov 18. Mucosal Immunol. 2016. PMID: 26577569 Free PMC article.
-
The innate immune responses of colonic epithelial cells to Trichuris muris are similar in mouse strains that develop a type 1 or type 2 adaptive immune response.Infect Immun. 2006 Nov;74(11):6280-6. doi: 10.1128/IAI.01609-05. Infect Immun. 2006. PMID: 17057095 Free PMC article.
Cited by
-
Influence of NOD2 Variants on Trichuris suis ova Treatment Outcome in Crohn's Disease.Front Pharmacol. 2018 Jul 16;9:764. doi: 10.3389/fphar.2018.00764. eCollection 2018. Front Pharmacol. 2018. PMID: 30061834 Free PMC article.
-
Microbial Sensing by Intestinal Myeloid Cells Controls Carcinogenesis and Epithelial Differentiation.Cell Rep. 2018 Aug 28;24(9):2342-2355. doi: 10.1016/j.celrep.2018.07.066. Cell Rep. 2018. PMID: 30157428 Free PMC article.
-
Organoids - New Models for Host-Helminth Interactions.Trends Parasitol. 2020 Feb;36(2):170-181. doi: 10.1016/j.pt.2019.10.013. Epub 2019 Nov 29. Trends Parasitol. 2020. PMID: 31791691 Free PMC article. Review.
-
Inflammation Regulation by Bacterial Molecular Patterns.Biomedicines. 2023 Jan 11;11(1):183. doi: 10.3390/biomedicines11010183. Biomedicines. 2023. PMID: 36672691 Free PMC article.
-
Differential Expression of Soluble Receptor for Advanced Glycation End-products in Mice Susceptible or Resistant to Chronic Colitis.Inflamm Bowel Dis. 2020 Feb 11;26(3):360-368. doi: 10.1093/ibd/izz311. Inflamm Bowel Dis. 2020. PMID: 31840738 Free PMC article.
References
-
- Crompton DWT. How Much Human Helminthiasis Is There in the World? The Journal of Parasitology. 1999;85(3):397–403. - PubMed
-
- Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv Parasitol. 2004;57:255–307. - PubMed
-
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427(6970):154–9. - PubMed
-
- Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8(3):193–204. - PubMed
-
- Rimoldi M, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nature Immunology. 2005;6(5):507–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

