Micro-a-fluidics ELISA for rapid CD4 cell count at the point-of-care
- PMID: 24448112
- PMCID: PMC3898414
- DOI: 10.1038/srep03796
Micro-a-fluidics ELISA for rapid CD4 cell count at the point-of-care
Abstract
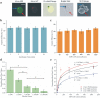
HIV has become one of the most devastating pathogens in human history. Despite fast progress in HIV-related basic research, antiretroviral therapy (ART) remains the most effective method to save AIDS patients' lives. Unfortunately, ART cannot be universally accessed, especially in developing countries, due to the lack of effective treatment monitoring diagnostics. Here, we present an inexpensive, rapid and portable micro-a-fluidic platform, which can streamline the process of an enzyme-linked immunosorbent assay (ELISA) in a fully automated manner for CD4 cell count. The micro-a-fluidic CD4 cell count is achieved by eliminating operational fluid flow via "moving the substrate", as opposed to "flowing liquid" in traditional ELISA or microfluidic methods. This is the first demonstration of capturing and detecting cells from unprocessed whole blood using the enzyme-linked immunosorbent assay (ELISA) in a microfluidic channel. Combined with cell phone imaging, the presented micro-a-fluidic ELISA platform holds great promise for offering rapid CD4 cell count to scale up much needed ART in resource-constrained settings. The developed system can be extended to multiple areas for ELISA-related assays.
Conflict of interest statement
U.D. is a founder of, and has an equity interest in, DxNow, a company that is developing microfluidic and imaging technologies for point-of-care diagnostic solutions. U.D.'s interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Figures



Similar articles
-
Emerging technologies for point-of-care management of HIV infection.Annu Rev Med. 2015;66:387-405. doi: 10.1146/annurev-med-092112-143017. Epub 2014 Nov 12. Annu Rev Med. 2015. PMID: 25423597 Review.
-
Enumeration of CD4+ T-cells using a portable microchip count platform in Tanzanian HIV-infected patients.PLoS One. 2011;6(7):e21409. doi: 10.1371/journal.pone.0021409. Epub 2011 Jul 6. PLoS One. 2011. PMID: 21754988 Free PMC article.
-
Near patient CD4 count in a hospitalized HIV patient population.Cytometry B Clin Cytom. 2017 Nov;92(6):451-455. doi: 10.1002/cyto.b.21248. Epub 2015 May 27. Cytometry B Clin Cytom. 2017. PMID: 25917935 Free PMC article.
-
Use of dried whole blood spots to measure CD4+ lymphocyte counts in HIV-1-infected patients.Lancet. 2003 Nov 1;362(9394):1459-60. doi: 10.1016/S0140-6736(03)14693-4. Lancet. 2003. PMID: 14602443
-
Thirty-five years of CD4 T-cell counting in HIV infection: From flow cytometry in the lab to point-of-care testing in the field.Cytometry B Clin Cytom. 2017 Nov;92(6):437-444. doi: 10.1002/cyto.b.21400. Epub 2016 Aug 3. Cytometry B Clin Cytom. 2017. PMID: 27406947 Review.
Cited by
-
Paper and flexible substrates as materials for biosensing platforms to detect multiple biotargets.Sci Rep. 2015 Mar 6;5:8719. doi: 10.1038/srep08719. Sci Rep. 2015. PMID: 25743880 Free PMC article.
-
Enzyme-catalyzed Ag Growth on Au Nanoparticle-assembled Structure for Highly Sensitive Colorimetric Immunoassay.Sci Rep. 2018 Apr 19;8(1):6290. doi: 10.1038/s41598-018-24664-w. Sci Rep. 2018. PMID: 29674713 Free PMC article.
-
Integrating Cell Phone Imaging with Magnetic Levitation (i-LEV) for Label-Free Blood Analysis at the Point-of-Living.Small. 2016 Mar 2;12(9):1222-1229. doi: 10.1002/smll.201501845. Epub 2015 Nov 2. Small. 2016. PMID: 26523938 Free PMC article.
-
Latent syphilis among inpatients in an urban area of China.Glob J Health Sci. 2014 Nov 30;7(3):249-53. doi: 10.5539/gjhs.v7n3p249. Glob J Health Sci. 2014. PMID: 25948433 Free PMC article.
-
Development of a C-reactive protein quantification method based on flow rate measurement of an ink solution pushed out by oxygen gas generated by catalase reaction.Mikrochim Acta. 2023 Dec 13;191(1):24. doi: 10.1007/s00604-023-06108-z. Mikrochim Acta. 2023. PMID: 38091091
References
-
- UNAIDS. UNAIDS Report on the global AIDS epidemic. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiolo... (Data accessed: 12/2013).
-
- Whitesides G. M. The origins and the future of microfluidics. Nature. 442, 368–373 (2006). - PubMed
-
- Yager P. et al. Microfluidic diagnostic technologies for global public health. Nature. 442, 412–418 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

