Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma
- PMID: 24448244
- PMCID: PMC4036226
- DOI: 10.1158/0008-5472.CAN-13-2050
Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma
Abstract
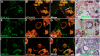
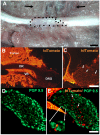
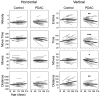
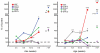
Perineural tumor invasion of intrapancreatic nerves, neurogenic inflammation, and tumor metastases along extrapancreatic nerves are key features of pancreatic malignancies. Animal studies show that chronic pancreatic inflammation produces hypertrophy and hypersensitivity of pancreatic afferents and that sensory fibers may themselves drive inflammation via neurogenic mechanisms. Although genetic mutations are required for cancer development, inflammation has been shown to be a precipitating event that can accelerate the transition of precancerous lesions to cancer. These observations led us to hypothesize that inflammation that accompanies early phases of pancreatic ductal adenocarcinoma (PDAC) would produce pathologic changes in pancreatic neurons and innervation. Using a lineage-labeled genetically engineered mouse model of PDAC, we found that pancreatic neurotrophic factor mRNA expression and sensory innervation increased dramatically when only pancreatic intraepithelial neoplasia were apparent. These changes correlated with pain-related decreases in exploratory behavior and increased expression of nociceptive genes in sensory ganglia. At later stages, cells of pancreatic origin could be found in the celiac and sensory ganglia along with metastases to the spinal cord. These results demonstrate that the nervous system participates in all stages of PDAC, including those that precede the appearance of cancer.
©2014 AACR.
Figures


References
-
- Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177–186.e1. - PubMed
-
- Ceyhan GO, Schäfer K-H, Kerscher AG, Rauch U, Demir IE, Kadihasanoglu M, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann. Surg. 2010;251:923–31. - PubMed
-
- Ma J, Jiang Y, Jiang Y, Sun Y, Zhao X. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J. Gastroenterol. Hepatol. 2008;23:1852–9. - PubMed
-
- Zeng Q, Cheng Y, Zhu Q, Yu Z, Wu X, Huang K, et al. The Relationship between Over-expression of Glial Cell-derived Neurotrophic Factor and Its RET Receptor with Progression and Prognosis of Human Pancreatic Cancer. J. Int. Med. Res. 2008;36:656–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000005/TR/NCATS NIH HHS/United States
- K08 DK088945/DK/NIDDK NIH HHS/United States
- DK063922/DK/NIDDK NIH HHS/United States
- R01 NS050758/NS/NINDS NIH HHS/United States
- T32 DK063922/DK/NIDDK NIH HHS/United States
- R03 NS075760/NS/NINDS NIH HHS/United States
- NS075760/NS/NINDS NIH HHS/United States
- DK088945/DK/NIDDK NIH HHS/United States
- R01 CA177857/CA/NCI NIH HHS/United States
- R01 NS033730/NS/NINDS NIH HHS/United States
- NS050758/NS/NINDS NIH HHS/United States
- CA177857/CA/NCI NIH HHS/United States
- NS033730/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

