Dissolution of the metal sensitizers Ni, Be, Cr in artificial sweat to improve estimates of dermal bioaccessibility
- PMID: 24448251
- PMCID: PMC4547829
- DOI: 10.1039/c3em00570d
Dissolution of the metal sensitizers Ni, Be, Cr in artificial sweat to improve estimates of dermal bioaccessibility
Abstract
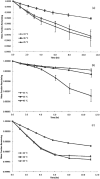
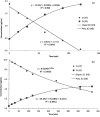
Dermal exposure to sensitizing metals is a serious occupational and public health problem. The usual approach to dermal exposure assessment is to process samples by chemical methods that use reactants to digest the metal particles and quantify the mass. In the case of dermal exposure assessment, these reactants are not representative of the skin surface film liquids and hence, may overestimate bioaccessibility. We hypothesize that the amount and form of sensitizer on a sample that leaches in a biological fluid, as can be estimated using artificial sweat, may be a more relevant metric for assessing health risks. Beryllium metal (Be), nickel metal (Ni), and chromium carbide (Cr3C2) particles were characterized and masses of sensitizing ions were measured using established reactant-assisted digestion procedures and extraction in artificial sweat under physiologically relevant conditions. Chromium ions released into artificial sweat were speciated to understand valence states. The ratios of the fraction of metal dissolved in artificial sweat relative to that dissolved by chemical-specific reactants were 1/2 (Be), 1/108 (Ni), and 1/2500 (Cr). The divalent Be and Ni cations were stable in artificial sweat over time (did not precipitate) whereas hexavalent chromium [Cr(VI)] ions decayed over time. Further analysis using speciated isotope dilution mass spectrometry revealed that the decay of Cr(VI) was accompanied by the formation of Cr(III) in the sweat model. Use of reactant-assisted analytical chemistry to quantify amounts of metal sensitizers on samples could overestimate biologically relevant exposure. In addition to mass, the valence state also influences penetration through the outer stratum corneum of the skin and is an important consideration when assessing exposure to complex sensitizers such as Cr which have multiple valence states with differing penetration efficiencies.
Figures


Similar articles
-
Particles, sweat, and tears: a comparative study on bioaccessibility of ferrochromium alloy and stainless steel particles, the pure metals and their metal oxides, in simulated skin and eye contact.Integr Environ Assess Manag. 2010 Jul;6(3):456-68. doi: 10.1002/ieam.66. Integr Environ Assess Manag. 2010. PMID: 20821707
-
Comparison of five artificial skin surface film liquids for assessing dermal bioaccessibility of metals in certified reference soils.Sci Total Environ. 2019 Nov 20;692:595-601. doi: 10.1016/j.scitotenv.2019.07.281. Epub 2019 Jul 18. Sci Total Environ. 2019. PMID: 31539967
-
Release of beryllium from beryllium-containing materials in artificial skin surface film liquids.Ann Occup Hyg. 2011 Jan;55(1):57-69. doi: 10.1093/annhyg/meq057. Epub 2010 Aug 20. Ann Occup Hyg. 2011. PMID: 20729394
-
Dissolution of materials in artificial skin surface film liquids.Toxicol In Vitro. 2006 Dec;20(8):1265-83. doi: 10.1016/j.tiv.2006.05.011. Epub 2006 Jun 15. Toxicol In Vitro. 2006. PMID: 16860531 Review.
-
[Occupational diseases caused by exposure to sensitizing metals].Sangyo Igaku. 1993 Mar;35(2):75-87. doi: 10.1539/joh1959.35.75. Sangyo Igaku. 1993. PMID: 8510347 Review. Japanese.
Cited by
-
Particle transfer and adherence to human skin compared with cotton glove and pre-moistened polyvinyl alcohol exposure sampling substrates.J Environ Sci Health A Tox Hazard Subst Environ Eng. 2021;56(5):585-598. doi: 10.1080/10934529.2021.1899524. Epub 2021 Mar 15. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2021. PMID: 33720803 Free PMC article.
-
Novel Insights into the Dermal Bioaccessibility and Human Exposure to Brominated Flame Retardant Additives in Microplastics.Environ Sci Technol. 2023 Jul 25;57(29):10554-10562. doi: 10.1021/acs.est.3c01894. Epub 2023 Jul 14. Environ Sci Technol. 2023. PMID: 37450894 Free PMC article.
-
Dermal bioaccessibility of flame retardants from indoor dust and the influence of topically applied cosmetics.J Expo Sci Environ Epidemiol. 2017 Jan;27(1):100-105. doi: 10.1038/jes.2015.84. Epub 2016 Jan 6. J Expo Sci Environ Epidemiol. 2017. PMID: 26732374
-
Updates in Metal Allergy: A Review of New Pathways of Sensitization, Exposure, and Treatment.Curr Allergy Asthma Rep. 2025 Jun 19;25(1):28. doi: 10.1007/s11882-025-01209-6. Curr Allergy Asthma Rep. 2025. PMID: 40536600 Review.
-
A comprehensive summary of disease variants implicated in metal allergy.J Toxicol Environ Health B Crit Rev. 2022 Aug 18;25(6):279-341. doi: 10.1080/10937404.2022.2104981. Epub 2022 Aug 16. J Toxicol Environ Health B Crit Rev. 2022. PMID: 35975293 Free PMC article. Review.
References
-
- Forte G, Petrucci F, Bocca B. Inflammation Allergy: Drug Targets. 2008;7:145–162. - PubMed
-
- Karlberg AT, Bergstrom MA, Borje A, Luthman K, Nilsson JL. Chern. Res. Toxicol. 2008;21:53–69. - PubMed
-
- Shelnutt SR, Goad P, Belsito DV. Grit. Rev. Toxicol. 2007;37:375–387. - PubMed
-
- Arts JH, Mommers C, de Heer C. Grit. Rev. Toxicol. 2006;36:219–251. - PubMed
-
- Aubin F. In: Measuring the Skin: Non-Invasive Investigations, Physiology, Normal Constants. Agache P, Humbert P, editors. Springer-Verlag; Germany: 2004. pp. 583–590.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

