Pregnancy as a cardiac stress model
- PMID: 24448313
- PMCID: PMC3941597
- DOI: 10.1093/cvr/cvu013
Pregnancy as a cardiac stress model
Abstract
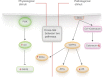
Cardiac hypertrophy occurs during pregnancy as a consequence of both volume overload and hormonal changes. Both pregnancy- and exercise-induced cardiac hypertrophy are generally thought to be similar and physiological. Despite the fact that there are shared transcriptional responses in both forms of cardiac adaptation, pregnancy results in a distinct signature of gene expression in the heart. In some cases, however, pregnancy can induce adverse cardiac events in previously healthy women without any known cardiovascular disease. Peripartum cardiomyopathy is the leading cause of non-obstetric mortality during pregnancy. To understand how pregnancy can cause heart disease, it is first important to understand cardiac adaptation during normal pregnancy. This review provides an overview of the cardiac consequences of pregnancy, including haemodynamic, functional, structural, and morphological adaptations, as well as molecular phenotypes. In addition, this review describes the signalling pathways responsible for pregnancy-induced cardiac hypertrophy and angiogenesis. We also compare and contrast cardiac adaptation in response to disease, exercise, and pregnancy. The comparisons of these settings of cardiac hypertrophy provide insight into pregnancy-associated cardiac adaptation.
Keywords: Hormones; Molecular signatures; Physiological cardiac hypertrophy; Pregnancy; Signalling pathways.
Figures



Comment in
-
Cardiac remodelling during pregnancy: whither the guinea pig?Cardiovasc Res. 2014 Oct 1;104(1):226-7. doi: 10.1093/cvr/cvu198. Epub 2014 Aug 25. Cardiovasc Res. 2014. PMID: 25155044 Free PMC article. No abstract available.
References
-
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. - PubMed
-
- McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34:255–262. - PubMed
-
- Gonzalez AMD, Osorio JC, Manlhiot C, Gruber D, Homma S, Mital S. Hypertrophy signaling during peripartum cardiac remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H3008–H3013. - PubMed
-
- Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol. 2010;300:H931–H942. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

