G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration
- PMID: 24448357
- PMCID: PMC3950854
- DOI: 10.1038/bjc.2013.822
G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration
Abstract
Background: Granulocyte colony-stimulating factor (G-CSF) is a pro-inflammatory cytokine that stimulates myeloid stem cell maturation, proliferation, and migration into circulation. Despite being a known growth factor, the impact of G-CSF on solid tumours has not been well examined. G-CSF receptor (G-CSFR) is expressed by some tumours, and thus the aim of this study was to examine the expression and impact of G-CSF and G-CSFR on gastrointestinal tumours.
Methods: In this study, G-CSF expression was examined in human gastric and colon tumours and by tumour-derived stromal myofibroblasts and carcinoma cells. G-CSFR expression was examined on carcinoma cells isolated from human tissues. The effects of G-CSF on gastric and colon carcinoma cell proliferation, migration, and signalling were examined.
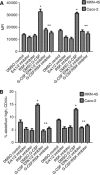
Results: G-CSFR was highly expressed in 90% of human gastric and colon carcinomas. G-CSF was also found to be highly produced by stromal myofibroblasts and carcinoma cells. Exposure of carcinoma cells to G-CSF led to increased proliferation and migration, and expansion of a sub-population of carcinoma cells expressing stem-like markers. These processes were dependent on ERK1/2 and RSK1 phosphorylation.
Conclusions: These data suggest that the G-CSF/R axis promotes gastric and colorectal cancer development and suggest they are potential tumour targets.
Figures






References
-
- Amariglio N, Jacob-Hirsch J, Shimoni A, Leiba M, Rechavi G, Nagler A. Changes in gene expression pattern following granulocyte colony-stimulating factor administration to normal stem cell sibling donors. Acta Haematol. 2007;117 (2:68–73. - PubMed
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet. 2001;357 (9255:539–545. - PubMed
-
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. - PubMed
-
- Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, Li C, Wang LP, Roby KF, Orsulic S, Connolly DC, Zhang Y, Montone K, Butzow R, Coukos G, Zhang L. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5 (4:e10277. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

