Poly (ADP-ribose) polymerase 1 transcriptional regulation: a novel crosstalk between histone modification H3K9ac and ETS1 motif hypomethylation in BRCA1-mutated ovarian cancer
- PMID: 24448423
- PMCID: PMC3960209
- DOI: 10.18632/oncotarget.1549
Poly (ADP-ribose) polymerase 1 transcriptional regulation: a novel crosstalk between histone modification H3K9ac and ETS1 motif hypomethylation in BRCA1-mutated ovarian cancer
Abstract
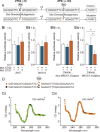
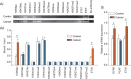
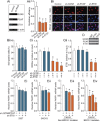
Poly (ADP-ribose) polymerase 1 (PARP1) plays a critical role in ovarian cancer progression. However, the epigenetic mechanism regulating PARP1 transcription remains largely unknown. Here, we show that the hypomethylated ETS1 motif is a key regulatory element for the PARP1 gene in BRCA1-mutated ovarian cancer. Mechanistically, the ETS1 motif hypomethylation-mediated increase of active histone marker H3K9ac and transcription factor ETS1 enrichment synergistically activates PARP1 transcription. Clinicopathological data indicate that a hypomethylated ETS1 motif was associated with high-grade tumors (P = 0.026) and pN1 (P = 0.002). Univariate survival analysis demonstrated an association between the hypomethylated ETS1 motif and an increased risk of death in BRCA1-mutated ovarian cancer patients. Our findings imply that the genetic (such as BRCA1 mutation) and epigenetic mechanisms (such as hypomethylated ETS1 motif, and histone modification H3K9ac and transcription factor ETS1 binding) are jointly involved in the malignant progression of PARP1-related ovarian cancer.
Figures

Similar articles
-
A novel crosstalk between BRCA1 and poly (ADP-ribose) polymerase 1 in breast cancer.Cell Cycle. 2014;13(21):3442-9. doi: 10.4161/15384101.2014.956507. Cell Cycle. 2014. PMID: 25485588 Free PMC article.
-
Promoter hypomethylation, especially around the E26 transformation-specific motif, and increased expression of poly (ADP-ribose) polymerase 1 in BRCA-mutated serous ovarian cancer.BMC Cancer. 2013 Feb 26;13:90. doi: 10.1186/1471-2407-13-90. BMC Cancer. 2013. PMID: 23442605 Free PMC article.
-
Regulation of DNA methyltransferase 1 transcription in BRCA1-mutated breast cancer: a novel crosstalk between E2F1 motif hypermethylation and loss of histone H3 lysine 9 acetylation.Mol Cancer. 2014 Feb 6;13:26. doi: 10.1186/1476-4598-13-26. Mol Cancer. 2014. PMID: 24502362 Free PMC article.
-
[Molecular mechanisms of regulaion of transcription by PARP1].Mol Biol (Mosk). 2015 Jan-Feb;49(1):99-113. Mol Biol (Mosk). 2015. PMID: 25916114 Review. Russian.
-
The roles of PARP1 in gene control and cell differentiation.Curr Opin Genet Dev. 2010 Oct;20(5):512-8. doi: 10.1016/j.gde.2010.06.001. Epub 2010 Jun 28. Curr Opin Genet Dev. 2010. PMID: 20591646 Free PMC article. Review.
Cited by
-
Genetic and Epigenetic Biomarkers Associated with Early Relapse in Pediatric Acute Lymphoblastic Leukemia: A Focused Bioinformatics Study on DNA-Repair Genes.Biomedicines. 2024 Aug 5;12(8):1766. doi: 10.3390/biomedicines12081766. Biomedicines. 2024. PMID: 39200230 Free PMC article.
-
High PARP-1 expression predicts poor survival in acute myeloid leukemia and PARP-1 inhibitor and SAHA-bendamustine hybrid inhibitor combination treatment synergistically enhances anti-tumor effects.EBioMedicine. 2018 Dec;38:47-56. doi: 10.1016/j.ebiom.2018.11.025. Epub 2018 Nov 22. EBioMedicine. 2018. PMID: 30472087 Free PMC article.
-
A novel crosstalk between BRCA1 and poly (ADP-ribose) polymerase 1 in breast cancer.Cell Cycle. 2014;13(21):3442-9. doi: 10.4161/15384101.2014.956507. Cell Cycle. 2014. PMID: 25485588 Free PMC article.
-
BRCA1 as a nicotinamide adenine dinucleotide (NAD)-dependent metabolic switch in ovarian cancer.Cell Cycle. 2014;13(16):2564-71. doi: 10.4161/15384101.2015.942208. Cell Cycle. 2014. PMID: 25486197 Free PMC article.
-
FLI1 regulates radiotherapy resistance in nasopharyngeal carcinoma through TIE1-mediated PI3K/AKT signaling pathway.J Transl Med. 2023 Feb 22;21(1):134. doi: 10.1186/s12967-023-03986-y. J Transl Med. 2023. PMID: 36814284 Free PMC article.
References
-
- Lech A, Daneva T, Pashova S, Gagov H, Crayton R, Kukwa W, Czarnecka AM, Szczylik C. Ovarian cancer as a genetic disease. Front Biosci. 2013;18:543–563. - PubMed
-
- Murphy SK. Targeting the epigenome in ovarian cancer. Future Oncol. 2012;8:151–164. - PubMed
-
- Seeber LM, van Diest PJ. Epigenetics in ovarian cancer. Methods Mol Biol. 2012;863:253–269. - PubMed
-
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-defcient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

