Single-cell technologies for monitoring immune systems
- PMID: 24448570
- PMCID: PMC4040085
- DOI: 10.1038/ni.2796
Single-cell technologies for monitoring immune systems
Abstract
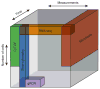
The complex heterogeneity of cells, and their interconnectedness with each other, are major challenges to identifying clinically relevant measurements that reflect the state and capability of the immune system. Highly multiplexed, single-cell technologies may be critical for identifying correlates of disease or immunological interventions as well as for elucidating the underlying mechanisms of immunity. Here we review limitations of bulk measurements and explore advances in single-cell technologies that overcome these problems by expanding the depth and breadth of functional and phenotypic analysis in space and time. The geometric increases in complexity of data make formidable hurdles for exploring, analyzing and presenting results. We summarize recent approaches to making such computations tractable and discuss challenges for integrating heterogeneous data obtained using these single-cell technologies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
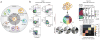


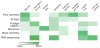
References
-
- Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4:648–655. - PubMed
-
- Chattopadhyay PK, et al. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat Med. 2006;12:972–977. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

