A highly reversible lithium metal anode
- PMID: 24448586
- PMCID: PMC5379181
- DOI: 10.1038/srep03815
A highly reversible lithium metal anode
Abstract
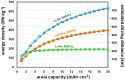
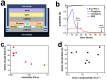
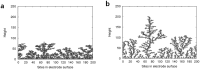
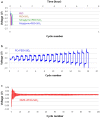
Lithium metal has shown a lot of promise for use as an anode material in rechargeable batteries owing to its high theoretical capacity. However, it does not meet the cycle life and safety requirements of rechargeable batteries owing to electrolyte decomposition and dendrite formation on the surfaces of the lithium anodes during electrochemical cycling. Here, we propose a novel electrolyte system that is relatively stable against lithium metal and mitigates dendritic growth. Systematic design methods that combined simulations, model-based experiments, and in situ analyses were employed to design the system. The reduction potential of the solvent, the size of the salt anions, and the viscosity of the electrolyte were found to be critical parameters determining the rate of dendritic growth. A lithium metal anode in contact with the designed electrolyte exhibited remarkable cyclability (more than 100 cycles) at a high areal capacity of 12 mAh cm(-2).
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Croce F., Appetecchi G. B., Persi L. & Scrosati B. Nanocomposite polymer electrolytes for lithium batteries. Nature 394, 456–458 (1998).
-
- Tarascon J.-M. & Armand M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001). - PubMed
-
- Bruce P. G., Freunberger S. A., Hardwick L. J. & Tarascon J.-M. Li-O2 and Li-S batteries with high energy storage. Nature Mater. 11, 19–29 (2012). - PubMed
-
- Ji X., Lee K. T. & Nazar L. F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nature Mater. 8, 500–506 (2009). - PubMed
-
- Jung H.-G., Hassoun J., Park J.-B., Sun Y.-K. & Scrosati B. An improved high-performance lithium-air battery. Nature Chem. 4, 579–585 (2012). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

