Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction
- PMID: 24448802
- PMCID: PMC3953278
- DOI: 10.1074/jbc.M113.545137
Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction
Abstract
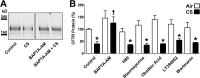
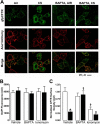
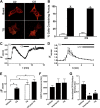
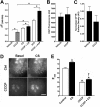
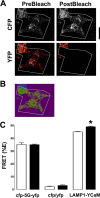
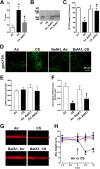
Chronic obstructive pulmonary disease affects 64 million people and is currently the fourth leading cause of death worldwide. Chronic obstructive pulmonary disease includes both emphysema and chronic bronchitis, and in the case of chronic bronchitis represents an inflammatory response of the airways that is associated with mucus hypersecretion and obstruction of small airways. Recently, it has emerged that exposure to cigarette smoke (CS) leads to an inhibition of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl(-) channel, causing airway surface liquid dehydration, which may play a role in the development of chronic bronchitis. CS rapidly clears CFTR from the plasma membrane and causes it to be deposited into aggresome-like compartments. However, little is known about the mechanism(s) responsible for the internalization of CFTR following CS exposure. Our studies revealed that CS triggered a rise in cytoplasmic Ca(2+) that may have emanated from lysosomes. Furthermore, chelation of cytoplasmic Ca(2+), but not inhibition of protein kinases/phosphatases, prevented CS-induced CFTR internalization. The macrolide antibiotic bafilomycin A1 inhibited CS-induced Ca(2+) release and prevented CFTR clearance from the plasma membrane, further linking cytoplasmic Ca(2+) and CFTR internalization. We hypothesize that CS-induced Ca(2+) release prevents normal sorting/degradation of CFTR and causes internalized CFTR to reroute to aggresomes. Our data provide mechanistic insight into the potentially deleterious effects of CS on airway epithelia and outline a hitherto unrecognized signaling event triggered by CS that may affect the long term transition of the lung into a hyper-inflammatory/dehydrated environment.
Keywords: Chronic Obstructive Pulmonary Disease (COPD); Cystic Fibrosis; Epithelium; Ion Channels; Lysosomes.
Figures

References
-
- Bartlett J. A., Fischer A. J., McCray P. B., Jr. (2008) Innate immune functions of the airway epithelium. Contrib. Microbiol. 15, 147–163 - PubMed
-
- Schmid A., Clunes L. A., Salathe M., Verdugo P., Dietl P., Davis C. W., Tarran R. (2011) Nucleotide-mediated airway clearance. Subcell. Biochem. 55, 95–138 - PubMed
-
- Com G., Clancy J. P. (2009) Adenosine receptors, cystic fibrosis, and airway hydration. Handb. Exp. Pharmacol. 363–381 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

