Diabetes and immunity to tuberculosis
- PMID: 24448841
- PMCID: PMC4213860
- DOI: 10.1002/eji.201344301
Diabetes and immunity to tuberculosis
Abstract
The dual burden of tuberculosis (TB) and diabetes has attracted much attention in the past decade as diabetes prevalence has increased dramatically in countries already afflicted with a high burden of TB. The confluence of these two major diseases presents a serious threat to global public health; at the same time it also presents an opportunity to learn more about the key elements of human immunity to TB that may be relevant to the general population. Some effects of diabetes on innate and adaptive immunity that are potentially relevant to TB defense have been identified, but have yet to be verified in humans and are unlikely to fully explain the interaction of these two disease states. This review provides an update on the clinical and epidemiological features of TB in the diabetic population and relates them to recent advances in understanding the mechanistic basis of TB susceptibility and other complications of diabetes. Issues that merit further investigation - such as geographic host and pathogen differences in the diabetes/TB interaction, the role of hyperglycemia-induced epigenetic reprogramming in immune dysfunction, and the impact of diabetes on lung injury and fibrosis caused by TB - are highlighted in this review.
Keywords: Bacterial infections; Diabetes; Host/pathogen interaction; Infectious disease; Tuberculosis.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Figures

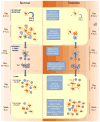
References
-
- Chen L, Magliano DJ, Zimmet PZL. The worldwide epidemiology of type 2 diabetes mellitus – present and future perspectives. Nat Rev Endocrinol. 2011;8:228–236. - PubMed
-
- Webb EA, Hesseling AC, Schaaf HS, Gie RP, Lombard CJ, Spitaels A, Delport S, et al. High prevalence of Mycobacterium tuberculosis infection and disease in children and adolescents with type 1 diabetes mellitus. Int J Tuberc Lung Dis. 2009;13:868–874. - PubMed
-
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

