Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer
- PMID: 24449231
- PMCID: PMC3927736
- DOI: 10.1200/JCO.2013.52.3696
Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer
Erratum in
- J Clin Oncol. 2014 May 1;32(13):1387
Abstract
Purpose: Prognostic models for overall survival (OS) for patients with metastatic castration-resistant prostate cancer (mCRPC) are dated and do not reflect significant advances in treatment options available for these patients. This work developed and validated an updated prognostic model to predict OS in patients receiving first-line chemotherapy.
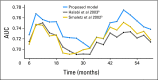
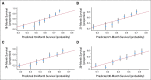
Methods: Data from a phase III trial of 1,050 patients with mCRPC were used (Cancer and Leukemia Group B CALGB-90401 [Alliance]). The data were randomly split into training and testing sets. A separate phase III trial served as an independent validation set. Adaptive least absolute shrinkage and selection operator selected eight factors prognostic for OS. A predictive score was computed from the regression coefficients and used to classify patients into low- and high-risk groups. The model was assessed for its predictive accuracy using the time-dependent area under the curve (tAUC).
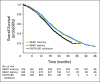
Results: The model included Eastern Cooperative Oncology Group performance status, disease site, lactate dehydrogenase, opioid analgesic use, albumin, hemoglobin, prostate-specific antigen, and alkaline phosphatase. Median OS values in the high- and low-risk groups, respectively, in the testing set were 17 and 30 months (hazard ratio [HR], 2.2; P < .001); in the validation set they were 14 and 26 months (HR, 2.9; P < .001). The tAUCs were 0.73 (95% CI, 0.70 to 0.73) and 0.76 (95% CI, 0.72 to 0.76) in the testing and validation sets, respectively.
Conclusion: An updated prognostic model for OS in patients with mCRPC receiving first-line chemotherapy was developed and validated on an external set. This model can be used to predict OS, as well as to better select patients to participate in trials on the basis of their prognosis.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



References
-
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. - PubMed
-
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

