Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer
- PMID: 24449235
- PMCID: PMC3927731
- DOI: 10.1200/JCO.2013.51.3226
Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer
Abstract
Purpose: Although guidelines recommend in-person counseling before BRCA1/BRCA2 gene testing, genetic counseling is increasingly offered by telephone. As genomic testing becomes more common, evaluating alternative delivery approaches becomes increasingly salient. We tested whether telephone delivery of BRCA1/2 genetic counseling was noninferior to in-person delivery.
Patients and methods: Participants (women age 21 to 85 years who did not have newly diagnosed or metastatic cancer and lived within a study site catchment area) were randomly assigned to usual care (UC; n = 334) or telephone counseling (TC; n = 335). UC participants received in-person pre- and post-test counseling; TC participants completed all counseling by telephone. Primary outcomes were knowledge, satisfaction, decision conflict, distress, and quality of life; secondary outcomes were equivalence of BRCA1/2 test uptake and costs of delivering TC versus UC.
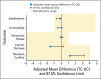
Results: TC was noninferior to UC on all primary outcomes. At 2 weeks after pretest counseling, knowledge (d = 0.03; lower bound of 97.5% CI, -0.61), perceived stress (d = -0.12; upper bound of 97.5% CI, 0.21), and satisfaction (d = -0.16; lower bound of 97.5% CI, -0.70) had group differences and confidence intervals that did not cross their 1-point noninferiority limits. Decision conflict (d = 1.1; upper bound of 97.5% CI, 3.3) and cancer distress (d = -1.6; upper bound of 97.5% CI, 0.27) did not cross their 4-point noninferiority limit. Results were comparable at 3 months. TC was not equivalent to UC on BRCA1/2 test uptake (UC, 90.1%; TC, 84.2%). TC yielded cost savings of $114 per patient.
Conclusion: Genetic counseling can be effectively and efficiently delivered via telephone to increase access and decrease costs.
Trial registration: ClinicalTrials.gov NCT00287898.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Is it time to embrace telephone genetic counseling in the oncology setting?J Clin Oncol. 2014 Mar 1;32(7):611-2. doi: 10.1200/JCO.2013.53.8975. Epub 2014 Jan 21. J Clin Oncol. 2014. PMID: 24449232 No abstract available.
References
-
- Mackenzie A, Patrick-Miller L, Bradbury AR. Controversies in communication of genetic risk for hereditary breast cancer. Breast J. 2009;15(suppl 1):S25–S32. - PubMed
-
- Robson ME, Storm CD, Weitzel J, et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. - PubMed
-
- American College of Surgeons. National Accreditation Program for Breast Centers, NAPBC Breast Center Components. Revised August 30, 2010. http://napbc-breast.org/standards/components.html.
-
- Commission on Cancer, American College of Surgeons. Cancer Program Standards 2012: Ensuring Patient-Centered Care. Version 1.2. http://www.facs.org/cancer/coc/programstandards2012.pdf.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

