Liquid biopsies: genotyping circulating tumor DNA
- PMID: 24449238
- PMCID: PMC4820760
- DOI: 10.1200/JCO.2012.45.2011
Liquid biopsies: genotyping circulating tumor DNA
Abstract
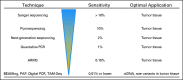
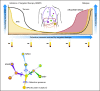
Genotyping tumor tissue in search of somatic genetic alterations for actionable information has become routine practice in clinical oncology. Although these sequence alterations are highly informative, sampling tumor tissue has significant inherent limitations; tumor tissue is a single snapshot in time, is subject to selection bias resulting from tumor heterogeneity, and can be difficult to obtain. Cell-free fragments of DNA are shed into the bloodstream by cells undergoing apoptosis or necrosis, and the load of circulating cell-free DNA (cfDNA) correlates with tumor staging and prognosis. Moreover, recent advances in the sensitivity and accuracy of DNA analysis have allowed for genotyping of cfDNA for somatic genomic alterations found in tumors. The ability to detect and quantify tumor mutations has proven effective in tracking tumor dynamics in real time as well as serving as a liquid biopsy that can be used for a variety of clinical and investigational applications not previously possible.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Mandel P, Metais P: Les acides nucleiques du plasma sanguin chez l'homme [in French] C R Seances Soc Biol Fil 142:241–243,1948 - PubMed
-
- Lo YM, Chiu RW: Genomic analysis of fetal nucleic acids in maternal blood Annu Rev Genomics Hum Genet 13:285–306,2012 - PubMed
-
- Lo YM Corbetta N Chamberlain PF, etal: Presence of fetal DNA in maternal plasma and serum Lancet 350:485–487,1997 - PubMed
-
- Lo YM Hjelm NM Fidler C, etal: Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma N Engl J Med 339:1734–1738,1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

