Zrf1 is required to establish and maintain neural progenitor identity
- PMID: 24449271
- PMCID: PMC3909791
- DOI: 10.1101/gad.228510.113
Zrf1 is required to establish and maintain neural progenitor identity
Abstract
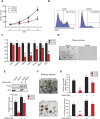
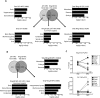
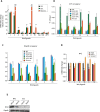
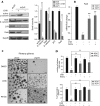
The molecular mechanisms underlying specification from embryonic stem cells (ESCs) and maintenance of neural progenitor cells (NPCs) are largely unknown. Recently, we reported that the Zuotin-related factor 1 (Zrf1) is necessary for chromatin displacement of the Polycomb-repressive complex 1 (PRC1). We found that Zrf1 is required for NPC specification from ESCs and that it promotes the expression of NPC markers, including the key regulator Pax6. Moreover, Zrf1 is essential to establish and maintain Wnt ligand expression levels, which are necessary for NPC self-renewal. Reactivation of proper Wnt signaling in Zrf1-depleted NPCs restores Pax6 expression and the self-renewal capacity. ESC-derived NPCs in vitro resemble most of the characteristics of the self-renewing NPCs located in the developing embryonic cortex, which are termed radial glial cells (RGCs). Depletion of Zrf1 in vivo impairs the expression of key self-renewal regulators and Wnt ligand genes in RGCs. Thus, we demonstrate that Zrf1 plays an essential role in NPC generation and maintenance.
Keywords: Wnt ligands; embryonic stem cell; neural specification.
Figures


References
-
- Aboody K, Capela A, Niazi N, Stern JH, Temple S 2011. Translating stem cell studies to the clinic for CNS repair: Current state of the art and the need for a Rosetta stone. Neuron 70: 597–613 - PubMed
-
- Aloia L, Di Stefano B, Di Croce L 2013. Polycomb complexes in stem cells and embryonic development. Development 140: 2525–2534 - PubMed
-
- Alvarez-Medina R, Le Dreau G, Ros M, Marti E 2009. Hedgehog activation is required upstream of Wnt signalling to control neural progenitor proliferation. Development 136: 3301–3309 - PubMed
-
- Aubert J, Dunstan H, Chambers I, Smith A 2002. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol 20: 1240–1245 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
