FDA approval of cardiac implantable electronic devices via original and supplement premarket approval pathways, 1979-2012
- PMID: 24449317
- PMCID: PMC4142419
- DOI: 10.1001/jama.2013.284986
FDA approval of cardiac implantable electronic devices via original and supplement premarket approval pathways, 1979-2012
Abstract
Importance: The US Food and Drug Administration (FDA) evaluates high-risk medical devices such as cardiac implantable electronic devices (CIEDs), including pacemakers, implantable cardioverter-defibrillators, and cardiac resynchronization therapy devices, via the premarket approval (PMA) process, during which manufacturers submit clinical data demonstrating safety and effectiveness. Subsequent changes to approved high-risk devices are implemented via "supplements," which may not require additional clinical testing.
Objective: To characterize the prevalence and characteristics of changes to CIEDs made through the PMA supplement process.
Design: Using the FDA's PMA database, we reviewed all CIEDs approved as original PMAs or supplements from 1979 through 2012. For each supplement, we collected the date approved, type of supplement (panel-track, 180-day, real-time, special, and 30-day notice), and the nature of the changes. We calculated the number of supplements approved per PMA and analyzed trends relating to different supplement regulatory categories over time. For supplements approved via the 180-day regulatory pathway, which often involve significant design changes, from 2010-2012, we identified how often additional clinical data were collected.
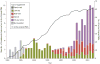
Results: From 1979-2012, the FDA approved 77 original and 5829 supplement PMA applications for CIEDs, with a median of 50 supplements per original PMA (interquartile range [IQR], 23-87). Excluding manufacturing changes that do not alter device design, the number of supplements approved each year was stable around a mean (SD) of 2.6 (0.9) supplements per PMA per year. Premarket approvals remained active via successive supplements over a median period of 15 years (IQR, 8-20), and 79% of the 77 original PMAs approved during our study period were the subject of at least 1 supplement in 2012. Thirty-seven percent of approved supplements involved a change to the device's design. Among 180-day supplements approved from 2010-2012, 23% (15/64) included new clinical data to support safety and effectiveness.
Conclusions and relevance: Many CIED models currently used by clinicians were approved via the PMA supplement process, not as original PMAs. Most new device models are deemed safe and effective without requiring new clinical data, reinforcing the importance of rigorous postapproval surveillance of these devices.
Conflict of interest statement
Figures

Comment in
-
Opening the FDA black box.JAMA. 2014 Jan 22-29;311(4):361-3. doi: 10.1001/jama.2013.283946. JAMA. 2014. PMID: 24449313 No abstract available.
-
Quality of evidence behind FDA approvals varies widely.BMJ. 2014 Jan 27;348:g1075. doi: 10.1136/bmj.g1075. BMJ. 2014. PMID: 24470635 No abstract available.
References
-
- Premarket approval (PMA) US Food and Drug Administration; [December, 26, 2013]. http://www.fda.gov/medicaldevices/deviceregulationandguidance/howtomarke...
-
- Medical Device Amendments of 1976 [Pub L No. 94-295] US Government Printing Office; [December, 26, 2013]. http://www.gpo.gov/fdsys/pkg/STATUTE-90/pdf/STATUTE-90-Pg539.pdf.
-
- Hauser RG, Almquist AK. Learning from our mistakes? testing new ICD technology. N Engl J Med. 2008;359(24):2517–2519. - PubMed
-
- Kay GN, Ellenbogen KA. An ICD lead advisory: a plea for more diligence and more data. Pacing Clin Electrophysiol. 2012;35(6):648–649. - PubMed
-
- Premarket approval (PMA) database. US Food and Drug Administration; [January 4, 2013]. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

