Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut
- PMID: 24449710
- PMCID: PMC3938613
- DOI: 10.1104/pp.113.228593
Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut
Abstract
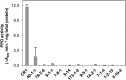
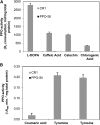
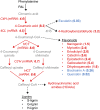
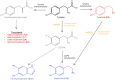
The enzyme polyphenol oxidase (PPO) catalyzes the oxidation of phenolic compounds into highly reactive quinones. Polymerization of PPO-derived quinones causes the postharvest browning of cut or bruised fruit, but the native physiological functions of PPOs in undamaged, intact plant cells are not well understood. Walnut (Juglans regia) produces a rich array of phenolic compounds and possesses a single PPO enzyme, rendering it an ideal model to study PPO. We generated a series of PPO-silenced transgenic walnut lines that display less than 5% of wild-type PPO activity. Strikingly, the PPO-silenced plants developed spontaneous necrotic lesions on their leaves in the absence of pathogen challenge (i.e. a lesion mimic phenotype). To gain a clearer perspective on the potential functions of PPO and its possible connection to cell death, we compared the leaf transcriptomes and metabolomes of wild-type and PPO-silenced plants. Silencing of PPO caused major alterations in the metabolism of phenolic compounds and their derivatives (e.g. coumaric acid and catechin) and in the expression of phenylpropanoid pathway genes. Several observed metabolic changes point to a direct role for PPO in the metabolism of tyrosine and in the biosynthesis of the hydroxycoumarin esculetin in vivo. In addition, PPO-silenced plants displayed massive (9-fold) increases in the tyrosine-derived metabolite tyramine, whose exogenous application elicits cell death in walnut and several other plant species. Overall, these results suggest that PPO plays a novel and fundamental role in secondary metabolism and acts as an indirect regulator of cell death in walnut.
Figures



References
-
- Belisario A, Scotton M, Santori A, Onofri S. (2008) Variability in the Italian population of Gnomonia leptostyla, homothallism and resistance of Juglans species to anthracnose. For Pathol 38: 129–145
-
- Belisario A, Zoina A, Pezza L, Luongo L. (1999) Susceptibility of species of Juglans to pathovars of Xanthomonas campestris. Eur J Forest Pathol 29: 75–80
-
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, B 57: 289–300
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

