Structural basis for the inhibition of the chromatin repressor BAHD1 by the bacterial nucleomodulin LntA
- PMID: 24449750
- PMCID: PMC3903274
- DOI: 10.1128/mBio.00775-13
Structural basis for the inhibition of the chromatin repressor BAHD1 by the bacterial nucleomodulin LntA
Abstract
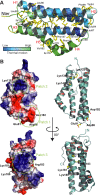
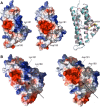
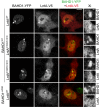
The nucleus has emerged as a key target for nucleomodulins, a family of effectors produced by bacterial pathogens to control host transcription or other nuclear processes. The virulence factor LntA from Listeria monocytogenes stimulates interferon responses during infection by inhibiting BAHD1, a nuclear protein involved in gene silencing by promoting heterochromatin formation. So far, whether the interaction between LntA and BAHD1 is direct and sufficient for inhibiting BAHD1 activity is unknown. Here, we functionally characterized the molecular interface between the two proteins in vitro and in transfected or infected human cells. Based on the known tridimensional structure of LntA, we identified a dilysine motif (K180/K181) in the elbow region of LntA and a central proline-rich region in BAHD1 as crucial for the direct LntA-BAHD1 interaction. To better understand the role played by the dilysine motif in the functionality of LntA, we solved the crystal structure of a K180D/K181D mutant to a 2.2-Å resolution. This mutant highlights a drastic redistribution of surface charges in the vicinity of a groove, which likely plays a role in nucleomodulin target recognition. Mutation of the strategic dilysine motif also abolished the recruitment of LntA to BAHD1-associated nuclear foci and impaired the LntA-mediated stimulation of interferon responses upon infection. Last, the strict conservation of residues K180 and K181 in LntA sequences from 188 L. monocytogenes strains of different serotypes and origins further supports their functional importance. Together, these results provide structural and functional details about the mechanism of inhibition of an epigenetic factor by a bacterial nucleomodulin.
Importance: Pathogens have evolved various strategies to deregulate the expression of host defense genes during infection, such as targeting nuclear proteins. LntA, a secreted virulence factor from the bacterium Listeria monocytogenes, stimulates innate immune responses by inhibiting a chromatin-associated repressor, BAHD1. This study reveals the structural features of LntA required for BAHD1 inhibition. LntA interacts directly with a central domain of BAHD1 via a surface patch of conserved positive charges, located nearby a groove on the elbow region of LntA. By demonstrating that this patch is required for LntA function, we provide a better understanding of the molecular mechanism allowing a bacterial pathogen to control host chromatin compaction and gene expression.
Figures


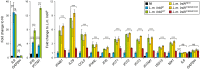
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

