IL-6 regulates extracellular matrix remodeling associated with aortic dilation in a fibrillin-1 hypomorphic mgR/mgR mouse model of severe Marfan syndrome
- PMID: 24449804
- PMCID: PMC3959679
- DOI: 10.1161/JAHA.113.000476
IL-6 regulates extracellular matrix remodeling associated with aortic dilation in a fibrillin-1 hypomorphic mgR/mgR mouse model of severe Marfan syndrome
Abstract
Background: Development of thoracic aortic aneurysms is the most significant clinical phenotype in patients with Marfan syndrome. An inflammatory response has been described in advanced stages of the disease. Because the hallmark of vascular inflammation is local interleukin-6 (IL-6) secretion, we explored the role of this proinflammatory cytokine in the formation of aortic aneurysms and rupture in hypomorphic fibrillin-deficient mice (mgR/mgR).
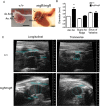
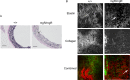
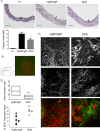
Methods and results: MgR/mgR mice developed ascending aortic aneurysms with significant dilation of the ascending aorta by 12 weeks (2.7 ± 0.1 and 1.3 ± 0.1 for mgR/mgR versus wild-type mice, respectively; P<0.001). IL-6 signaling was increased in mgR/mgR aortas measured by increases in IL-6 and SOCS3 mRNA transcripts (P<0.05) and in cytokine secretion of IL-6, MCP-1, and GM-CSF (P<0.05). To investigate the role of IL-6 signaling, we generated mgR homozygous mice with IL-6 deficiency (DKO). The extracellular matrix of mgR/mgR mice showed significant disruption of elastin and the presence of dysregulated collagen deposition in the medial-adventitial border by second harmonic generation multiphoton autofluorescence microscopy. DKO mice exhibited less elastin and collagen degeneration than mgR/mgR mice, which was associated with decreased activity of matrix metalloproteinase-9 and had significantly reduced aortic dilation (1.0 ± 0.1 versus 1.6 ± 0.2 mm change from baseline, DKO versus mgR/mgR, P<0.05) that did not affect rupture and survival.
Conclusion: Activation of IL-6-STAT3 signaling contributes to aneurysmal dilation in mgR/mgR mice through increased MMP-9 activity, aggravating extracellular matrix degradation.
Keywords: Marfan syndrome; extracellular matrix; interleukin‐6; matrix metalloproteinases; mgR; thoracic aortic aneurysms and dissections; vascular inflammation.
Figures



References
-
- Dietz HC, Loeys B, Carta L, Ramirez F. Recent progress towards a molecular understanding of Marfan syndrome. Am J Med Genet C Semin Med Genet. 2005; 139C:4-9 - PubMed
-
- Williams A, Davies S, Stuart AG, Wilson DG, Fraser AG. Medical treatment of Marfan syndrome: a time for change. Heart. 2008; 94:414-421 - PubMed
-
- Yetman AT. Cardiovascular pharmacotherapy in patients with Marfan syndrome. Am J Cardiovasc Drugs. 2007; 7:117-126 - PubMed
-
- Hirata K, Triposkiadis F, Sparks E, Bowen J, Wooley CF, Boudoulas H. The Marfan syndrome: abnormal aortic elastic properties. J Am Coll Cardiol. 1991; 18:57-63 - PubMed
-
- Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin‐1 (FBN1) in the Marfan syndrome and related disorders. Hum Mol Genet. 1995; 4 Spec No:1799-1809 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

