Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial
- PMID: 24449819
- PMCID: PMC4067050
- DOI: 10.1161/CIRCRESAHA.114.302854
Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial
Abstract
Rationale: Transendocardial stem cell injection (TESI) with mesenchymal stem cells improves remodeling in chronic ischemic cardiomyopathy, but the effect of the injection site remains unknown.
Objective: To address whether TESI exerts its effects at the site of injection only or also in remote areas, we hypothesized that segmental myocardial scar and segmental ejection fraction improve to a greater extent in injected than in noninjected segments.
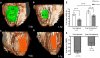
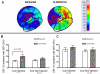
Methods and results: Biplane ventriculographic and endocardial tracings were recorded. TESI was guided to 10 sites in infarct-border zones. Sites were mapped according to the 17-myocardial segment model. As a result, 510 segments were analyzed in 30 patients before and 13 months after TESI. Segmental early enhancement defect (a measure of scar size) was reduced by TESI in both injected (-43.7 ± 4.4%; n=95; P<0.01) and noninjected segments (-25.1 ± 7.8%; n=148; P<0.001; between-group comparison P<0.05). Conversely, segmental ejection fraction (a measure of contractile performance) improved in injected scar segments (19.9 ± 3.3-26.3 ± 3.5%; P=0.003) but not in noninjected scar segments (21.3 ± 2.6-23.5 ± 3.2%; P=0.20; between-group comparison P<0.05). Furthermore, segmental ejection fraction in injected scar segments improved to a greater degree in patients with baseline segmental ejection fraction <20% (12.1 ± 1.2-19.9 ± 2.7%; n=18; P=0.003), versus <20% (31.7 ± 3.4-35.5 ± 3.3%; n=12; P=0.33, between-group comparison P<0.0001).
Conclusions: These findings illustrate a dichotomy in regional responses to TESI. Although scar size reduction was evident in all scar segments, scar size reduction and ventricular functional responses preferentially occurred at the sites of TESI versus non-TESI sites. Furthermore, improvement was greatest when segmental left ventricular dysfunction was severe.
Keywords: cells; magnetic resonance imaging; myocardial infarction; tomography.
Figures


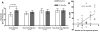
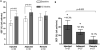
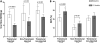
Comment in
-
PROMETHEUS and POSEIDON: Harnessing the power of advanced cardiac imaging.Circ Res. 2014 Apr 11;114(8):1222-4. doi: 10.1161/CIRCRESAHA.114.303792. Circ Res. 2014. PMID: 24723652 Free PMC article. No abstract available.
References
-
- Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. - PMC - PubMed
-
- Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. - PMC - PubMed
-
- Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. - PMC - PubMed
-
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA : the journal of the American Medical Association. 2012;308:2369–2379. - PMC - PubMed
-
- Dib N, Menasche P, Bartunek JJ, Zeiher AM, Terzic A, Chronos NA, Henry TD, Peters NS, Fernandez-Aviles F, Yacoub M, Sanborn TA, Demaria A, Schatz RA, Taylor DA, Fuchs S, Itescu S, Miller LW, Dinsmore JH, Dangas GD, Popma JJ, Hall JL, Holmes DR., Jr. Recommendations for successful training on methods of delivery of biologics for cardiac regeneration: A report of the international society for cardiovascular translational research. JACC Cardiovasc Interv. 2010;3:265–275. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

